|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000360 |
|---|
|
Identification |
|---|
| Name: |
Diaminopimelic acid |
|---|
| Description: | Diaminopimelic acid (DAP) is an amino acid, representing an epsilon-carboxy derivative of lysine. DAP found in the cell walls of Pseudomonas aeruginosa and is a component of peptidoglycan of Gram-negative bacteria. |
|---|
|
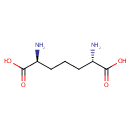
Structure |
|
|---|
| Synonyms: | - ( (R*,S*)-2,6-diamino-Heptanedioate
- ( (R*,S*)-2,6-diamino-Heptanedioic acid
- (2R,6S)-2,6-diamino-heptanedioate
- (2R,6S)-2,6-diamino-heptanedioic acid
- (R*,S*)-2,6-diamino-Heptanedioate
- (R*,S*)-2,6-diamino-Heptanedioic acid
- 2,6-Diamino-Heptanedioate
- 2,6-Diamino-Heptanedioic acid
- 2,6-Diaminoheptanedioate
- 2,6-Diaminoheptanedioic acid
- 2,6-Diaminopimelate
- 2,6-Diaminopimelic acid
- A,a'-Diaminopimelate
- A,a'-Diaminopimelic acid
- A,e-Diaminopimelate
- A,e-Diaminopimelic acid
- D,L-Diaminopimelate
- D,L-Diaminopimelic acid
- D,L-Meso-diaminoheptanedioate
- D,L-Meso-diaminoheptanedioic acid
- DAPA
- Diaminopimelate
- Diaminopimelic acid
- Dl-2,6-Diaminoheptanedioate
- Dl-2,6-Diaminoheptanedioic acid
- DL-2,6-Diaminopimelate
- DL-2,6-Diaminopimelic acid
- DPA
- L,L-2,6-Diaminoheptanedioate
- L,L-2,6-Diaminoheptanedioic acid
- L,L-2,6-Diaminopimelate
- L,L-2,6-Diaminopimelic acid
- L,L-A2pm
- L,L-DAP
- L,L-Diaminopimelate
- L,L-Diaminopimelic acid
- LL-2,6-diaminoheptanedioate
- LL-2,6-diaminoheptanedioic acid
- LL-2,6-Diaminopimelate
- LL-2,6-Diaminopimelic acid
- meso-1-a,epsilon-Diaminopimelate
- meso-1-a,epsilon-Diaminopimelic acid
- Meso-1-alpha,epsilon-diaminopimelate
- Meso-1-alpha,epsilon-diaminopimelic acid
- meso-1-α,epsilon-Diaminopimelate
- meso-1-α,epsilon-Diaminopimelic acid
- Meso-2,6-diamino-Heptanedioate
- Meso-2,6-diamino-Heptanedioic acid
- Meso-2,6-diaminoheptanedioate
- Meso-2,6-diaminoheptanedioic acid
- meso-a,Alpha'-diaminopimelate
- meso-a,Alpha'-diaminopimelic acid
- meso-a,epsilon-Diaminopimelate
- meso-a,epsilon-Diaminopimelic acid
- Meso-alpha,alpha'-Diaminopimelate
- Meso-alpha,alpha'-Diaminopimelic acid
- Meso-alpha,epsilon-Diaminopimelate
- Meso-alpha,epsilon-Diaminopimelic acid
- Meso-diaminoheptanedioate
- Meso-diaminoheptanedioic acid
- Meso-diaminopimelate
- Meso-diaminopimelic acid
- meso-α,Alpha'-diaminopimelate
- meso-α,Alpha'-diaminopimelic acid
- meso-α,epsilon-Diaminopimelate
- meso-α,epsilon-Diaminopimelic acid
|
|---|
|
Chemical Formula: |
C7H14N2O4 |
|---|
| Average Molecular Weight: |
190.1971 |
|---|
| Monoisotopic Molecular
Weight: |
190.095356946 |
|---|
| InChI Key: |
GMKMEZVLHJARHF-WHFBIAKZSA-N |
|---|
| InChI: | InChI=1S/C7H14N2O4/c8-4(6(10)11)2-1-3-5(9)7(12)13/h4-5H,1-3,8-9H2,(H,10,11)(H,12,13)/t4-,5-/m0/s1 |
|---|
| CAS
number: |
583-93-7 |
|---|
| IUPAC Name: | (2S,6S)-2,6-diaminoheptanedioic acid |
|---|
|
Traditional IUPAC Name: |
diamino-pimelic acid |
|---|
| SMILES: | N[C@@H](CCC[C@H](N)C(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- L-alpha-amino acid
- Medium-chain fatty acid
- Amino fatty acid
- Fatty acyl
- Fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
300 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-0umi-1950000000-749403a0eb6d05c7903e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-0uk9-1980000000-1b092f9141e7549bee24 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004l-0900000000-d85213bd7ac426f10cd7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001i-9100000000-add590f1b216bdb40334 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-001i-9000000000-de98ed3bb8c48b6f9852 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Borruat G, Roten CA, Fay LB, Karamata D: A high-performance liquid chromatography method for the detection of diaminopimelic acid in urine. Anal Biochem. 2001 Apr 1;291(1):11-6. Pubmed: 11262151
- Hamaker BR, Rivera K, Morales E, Graham GG: Effect of dietary fiber and starch on fecal composition in preschool children consuming maize, amaranth, or cassava flours. J Pediatr Gastroenterol Nutr. 1991 Jul;13(1):59-66. Pubmed: 1656007
- Iida S, Taniguchi H, Kageyama A, Yazawa K, Chibana H, Murata S, Nomura F, Kroppenstedt RM, Mikami Y: Gordonia otitidis sp. nov., isolated from a patient with external otitis. Int J Syst Evol Microbiol. 2005 Sep;55(Pt 5):1871-6. Pubmed: 16166681
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Ladesic B, Tomasic J, Kveder S, Hrsak I: The metabolic fate of 14C-labeled immunoadjuvant peptidoglycan monomer. II. In vitro studies. Biochim Biophys Acta. 1981 Nov 18;678(1):12-7. Pubmed: 6118181
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Gao, Yong. Synthesis of diaminopimelic acid (DAP) and analogues: mechanistic studies on DAP aminotransferase, epimerase and dehydrogenase. (1998), 166 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|