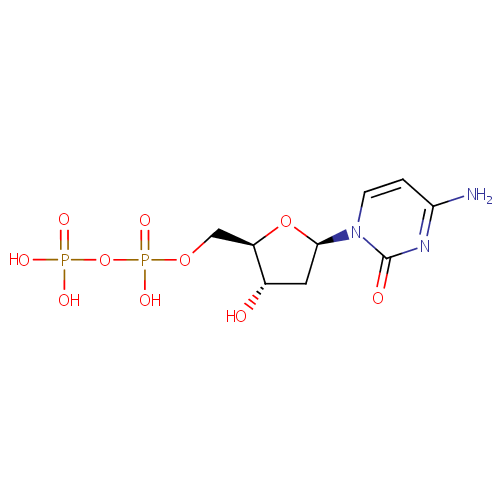
dCDP (PAMDB000307)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000307 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | dCDP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | dCDP or Deoxycytidine 5'-diphosphate (dCDP) is a nucleoside diphosphate. It is related to the common nucleic acid CTP, or cytidine triphosphate, with the -OH (hydroxyl) group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy- part of the name), and with one fewer phosphoryl group than CTP .dCDP is a product and competitive inhibitor of ribonucleoside-diphosphate reductase (EC 1.17.4.1) from Pseudomonas aeruginosa. Structural studies indicate the base is in anti conformation and the sugar in S-type puckering, when bound either to the complete enzyme complex or to the large protein subunit alone. [PMID: 8019775] Ribonucleoside-diphosphate reductase is very tightly controlled by a variety of allosteric effectors. The enzyme has different regions of it that act differently on allosteric regulators. At the activity site, dATP is a general inhibitor for all substrates and ATP is an activator. Binding of nucleotides at the specificity site further controls the activity of the enzyme towards different substrates in order to maintain an appropriate balance of all deoxynucleotides for DNA synthesis. The dNDPs produced by the enzyme are then phosphorylated to dNTPs by kinases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H15N3O10P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 387.177 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 387.023266739 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | FTDHDKPUHBLBTL-SHYZEUOFSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H15N3O10P2/c10-7-1-2-12(9(14)11-7)8-3-5(13)6(21-8)4-20-24(18,19)22-23(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H2,10,11,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 800-73-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [({[(2R,3S,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | dCDP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=NC(=O)N(C=C1)[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(O)=O)O1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleosides. These are compounds consisting of a pyrimidine linked to a ribose which lacks a hydroxyl group at position 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine 2'-deoxyribonucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine 2'-deoxyribonucleosides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | CDP + Reduced Thioredoxin > dCDP + Water + Oxidized Thioredoxin CDP + glutaredoxin > dCDP + glutaredoxin + Water Adenosine triphosphate + dCDP <> ADP + dCTP Adenosine triphosphate + dCMP <> ADP + dCDP dCDP + Thioredoxin disulfide + Water <> Thioredoxin + CDP dCTP + Uridine <> dCDP + Uridine 5'-monophosphate dCTP + Cytidine <> dCDP + Cytidine monophosphate Adenosine triphosphate + dCMP + Uridine 5'-monophosphate <> ADP + dCDP + Uridine 5'-diphosphate dCDP + Adenosine triphosphate > Adenosine diphosphate + dCTP + ADP CDP + a reduced NrdH glutaredoxin-like protein > Water + dCDP + an oxidized NrdH glutaredoxin-like protein | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Nara, Takashi; Misawa, Masanaru. Bacterial phosphorylation of 5'-deoxycytidine monophosphate to di-or triphosphate. Jpn. Tokkyo Koho (1971), 2 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidation-reduction process
- Specific function:
- Provides the precursors necessary for DNA synthesis. Catalyzes the biosynthesis of deoxyribonucleotides from the corresponding ribonucleotides. R1 contains the binding sites for both substrates and allosteric effectors and carries out the actual reduction of the ribonucleotide. It also provides redox- active cysteines
- Gene Name:
- nrdA
- Locus Tag:
- PA1156
- Molecular weight:
- 107.1 kDa
Reactions
| 2'-deoxyribonucleoside diphosphate + thioredoxin disulfide + H(2)O = ribonucleoside diphosphate + thioredoxin. |
- General function:
- Involved in cytidylate kinase activity
- Specific function:
- ATP, dATP, and GTP are equally effective as phosphate donors. CMP and dCMP are the best phosphate acceptors
- Gene Name:
- cmk
- Locus Tag:
- PA3163
- Molecular weight:
- 24.6 kDa
Reactions
| ATP + (d)CMP = ADP + (d)CDP. |
- General function:
- Involved in nucleoside diphosphate kinase activity
- Specific function:
- Major role in the synthesis of nucleoside triphosphates other than ATP. The ATP gamma phosphate is transferred to the NDP beta phosphate via a ping-pong mechanism, using a phosphorylated active-site intermediate
- Gene Name:
- ndk
- Locus Tag:
- PA3807
- Molecular weight:
- 15.6 kDa
Reactions
| ATP + nucleoside diphosphate = ADP + nucleoside triphosphate. |
- General function:
- Involved in ATP binding
- Specific function:
- Catalyzes the reversible transfer of the terminal phosphate group between ATP and AMP. This small ubiquitous enzyme involved in the energy metabolism and nucleotide synthesis, is essential for maintenance and cell growth
- Gene Name:
- adk
- Locus Tag:
- PA3686
- Molecular weight:
- 23.1 kDa
Reactions
| ATP + AMP = 2 ADP. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Provides the precursors necessary for DNA synthesis. Catalyzes the biosynthesis of deoxyribonucleotides from the corresponding ribonucleotides. R2 contains the tyrosyl radical required for catalysis
- Gene Name:
- nrdB
- Locus Tag:
- PA1155
- Molecular weight:
- 47.4 kDa
Reactions
| 2'-deoxyribonucleoside diphosphate + thioredoxin disulfide + H(2)O = ribonucleoside diphosphate + thioredoxin. |
- General function:
- Involved in electron carrier activity
- Specific function:
- Monothiol glutaredoxin involved in the biogenesis of iron-sulfur clusters (Probable)
- Gene Name:
- grxD
- Locus Tag:
- PA3533
- Molecular weight:
- 11.8 kDa
- General function:
- Involved in electron carrier activity
- Specific function:
- The disulfide bond functions as an electron carrier in the glutathione-dependent synthesis of deoxyribonucleotides by the enzyme ribonucleotide reductase. In addition, it is also involved in reducing some disulfides in a coupled system with glutathione reductase
- Gene Name:
- grxC
- Locus Tag:
- PA5129
- Molecular weight:
- 9.2 kDa
- General function:
- Involved in electron carrier activity
- Specific function:
- Participates in various redox reactions through the reversible oxidation of its active center dithiol to a disulfide and catalyzes dithiol-disulfide exchange reactions
- Gene Name:
- trxA
- Locus Tag:
- PA5240
- Molecular weight:
- 11.9 kDa