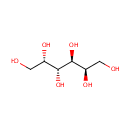
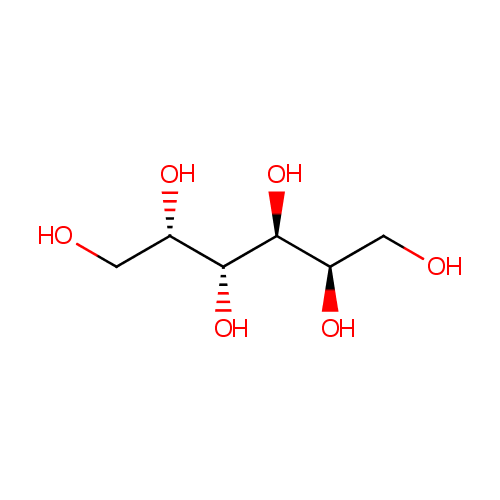
| References: |
- Airey CM, Price DE, Kemp JV, Perkins CM, Wales JK: The effect of aldose reductase inhibition on erythrocyte polyols and galactitol accumulation in diabetic patients. Diabet Med. 1989 Dec;6(9):804-8. Pubmed: 2533041
- Allen JT, Holton JB, Gillett MG: Gas-liquid chromatographic determination of galactitol in amniotic fluid for possible use in prenatal diagnosis of galactosaemia. Clin Chim Acta. 1981 Feb 19;110(1):59-63. Pubmed: 7214715
- Arola H, Sillanaukee P, Aine E, Koivula T, Isokoski M: Galactitol is not a cause of senile cataract. Graefes Arch Clin Exp Ophthalmol. 1992;230(3):240-2. Pubmed: 1597290
- Berry GT, Hunter JV, Wang Z, Dreha S, Mazur A, Brooks DG, Ning C, Zimmerman RA, Segal S: In vivo evidence of brain galactitol accumulation in an infant with galactosemia and encephalopathy. J Pediatr. 2001 Feb;138(2):260-2. Pubmed: 11174626
- Berry GT, Palmieri M, Gross KC, Acosta PB, Henstenburg JA, Mazur A, Reynolds R, Segal S: The effect of dietary fruits and vegetables on urinary galactitol excretion in galactose-1-phosphate uridyltransferase deficiency. J Inherit Metab Dis. 1993;16(1):91-100. Pubmed: 8487507
- Budde M, Gusek-Schneider GC, Junemann A, Jansen F, Shin YS: [Familial cataract in plasma galactitol increase without known enzyme defect] Klin Monatsbl Augenheilkd. 1999 Oct;215(4):255-7. Pubmed: 10572890
- Ficicioglu C, Yager C, Segal S: Galactitol and galactonate in red blood cells of children with the Duarte/galactosemia genotype. Mol Genet Metab. 2005 Feb;84(2):152-9. Epub 2004 Dec 9. Pubmed: 15670721
- Jakobs C, Schweitzer S, Dorland B: Galactitol in galactosemia. Eur J Pediatr. 1995;154(7 Suppl 2):S50-2. Pubmed: 7671965
- Jakobs C, Warner TG, Sweetman L, Nyhan WL: Stable isotope dilution analysis of galactitol in amniotic fluid: an accurate approach to the prenatal diagnosis of galactosemia. Pediatr Res. 1984 Aug;18(8):714-8. Pubmed: 6433315
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Ning C, Reynolds R, Chen J, Yager C, Berry GT, McNamara PD, Leslie N, Segal S: Galactose metabolism by the mouse with galactose-1-phosphate uridyltransferase deficiency. Pediatr Res. 2000 Aug;48(2):211-7. Pubmed: 10926297
- Ning C, Segal S: Plasma galactose and galactitol concentration in patients with galactose-1-phosphate uridyltransferase deficiency galactosemia: determination by gas chromatography/mass spectrometry. Metabolism. 2000 Nov;49(11):1460-6. Pubmed: 11092512
- Pettit BR, King GS, Blau K: The analysis of hexitols in biological fluid by selected ion monitoring. Biomed Mass Spectrom. 1980 Jul;7(7):309-13. Pubmed: 7448335
- Roboz J, Kappatos DC, Greaves J, Holland JF: Determination of polyols in serum by selected ion monitoring. Clin Chem. 1984 Oct;30(10):1611-5. Pubmed: 6434200
- Schwarz HP, Schaefer T, Bachmann C: Galactose and galactitol in the urine of children with compound heterozygosity for Duarte variant and classical galactosemia (GtD/gt) after an oral galactose load. Clin Chem. 1985 Mar;31(3):420-2. Pubmed: 3971562
- Shetty HU, Holloway HW, Rapoport SI: Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem. 1995 Jan 1;224(1):279-85. Pubmed: 7710082
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|