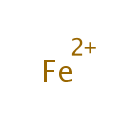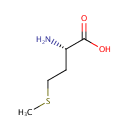Search Results for compounds
Searching compounds for
returned 4373 results.
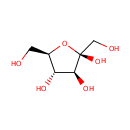
D-Fructose (PAMDB000167)
IUPAC:
(2R,3S,4S,5R)-2,5-bis(hydroxymethyl)oxolane-2,3,4-triol
CAS: 53188-23-1
Description: Fructose, or levulose, is a levorotatory monosaccharide and an isomer of glucose (C6H12O6). The chemical composition of fructose is (C6H12O6). Pure fructose has a sweet taste similar to cane sugar, but with a "fruity" aroma. Although fructose is a hexose (6 carbon sugar), it generally exists as a 5-member hemiketal ring (a furanose). This structure is responsible for the long metabolic pathway and high reactivity compared to glucose. Fructose is found in many foods. Honey; tree fruits; berries; melons; and some root vegetables, such as beets, sweet potatoes, parsnips and onions, contain fructose, usually in combination with sucrose and glucose. Fructose is also derived from the digestion of sucrose, a disaccharide consisting of glucose and fructose that is broken down by enzymes during digestion. Fructose is the sweetest naturally occurring sugar, estimated to be twice as sweet as sucrose. Fructose is a reducing sugar, as are all monosaccharides.
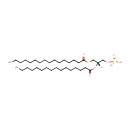
PA(16:0/16:0) (PAMDB000169)
IUPAC:
[(2R)-2,3-bis(hexadecanoyloxy)propoxy]phosphonic acid
CAS: 7091-44-3
Description: PA(16:0/16:0) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(16:0/16:0), in particular, consists of one chain of palmitic acid at the C-1 position and one chain of palmitic acid at the C-2 position. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. Phospholipase Ds (PLDs), which catalyze the conversion of glycerolphospholipids, particularly phosphatidylcholine, to PAs and the conversion of N-arachidonoyl-phosphatidylethanolamine (NAPE) to anandamide and PAs are activated by several inflammatory mediators including bradykinin, ATP and glutamate. Circumstantial evidence that PAs are converted to DAGs. (PMID: 12618218, 16185776).
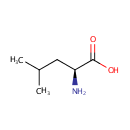
L-Leucine (PAMDB000170)
IUPAC:
(2S)-2-amino-4-methylpentanoic acid
CAS: 61-90-5
Description: Leucine (abbreviated as Leu or L) is an amino acid with the chemical formula HO2CCH(NH2)CH2CH(CH3)2. It is synthesized in plants and microorganisms via several steps starting from pyruvic acid. The initial part of the pathway also leads to valine. The intermediate alpha-ketovalerate is converted to alpha-isopropylmalate and then beta-isopropylmalate, which is dehydrogenated to alpha-ketoisocaproate, which in the final step undergoes reductive amination.
Iron (PAMDB000172)
IUPAC:
??-iron(2+) ion
CAS: 7439-89-6
Description: Iron is a chemical element with the symbol Fe and atomic number 26. Iron makes up 5% of the Earth's crust and is second in abundance to aluminium among the metals and fourth in abundance among the elements. Iron (as Fe2+, ferrous ion) is a necessary trace element used by all known living organisms. Iron-containing enzymes, usually containing heme prosthetic groups, participate in catalysis of oxidation reactions in biology, and in transport of a number of soluble gases. Its chief functions are in the transport of oxygen to tissue (hemoglobin) and in cellular oxidation mechanisms. Inorganic iron involved in redox reactions is also found in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. A class of non-heme iron proteins is responsible for a wide range of functions such as ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis) and purple acid phosphatase (hydrolysis of phosphate esters). When the body is fighting a bacterial infection, the body sequesters iron inside of cells (mostly stored in the storage molecule ferritin) so that it cannot be used by bacteria. Iron may promote both growth of Pseudomonas aeruginosa.
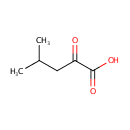
Ketoleucine (PAMDB000174)
IUPAC:
4-methyl-2-oxopentanoic acid
CAS: 816-66-0
Description: Ketoleucine belongs to the class of Branched Fatty Acids. These are fatty acids containing a branched chain. (inferred from compound structure) Ketoleucine is also known by names such as 4-methyl-2-oxopentanoic acid and 2-Oxoisocaproic acid. (PubChem) In Pseudomonas aeruginosa, the enzyme branched-chain amino acid aminotransferase (EC:2.6.1.42) catalyses the reversible conversion between ketoleucine and L-leucine. (KEGG)
L-Methionine (PAMDB000175)
IUPAC:
(2S)-2-amino-4-(methylsulfanyl)butanoic acid
CAS: 63-68-3
Description: L-Methionine, in addition to being a substrate for protein synthesis, is an intermediate in transmethylation reactions, serving as the major methyl group donor, including the methyl groups for DNA and RNA intermediates. Methionine is a methyl acceptor for 5-methyltetrahydrofolate-homocysteine methyl transferase (methionine synthase), the only reaction that allows for the recycling of this form of folate, and is also a methyl acceptor for the catabolism of betaine. Methionine is also required for synthesis of cysteine. Methionine is accepted as the metabolic precursor for cysteine. Only the sulfur atom from methionine is transferred to cysteine; the carbon skeleton of cysteine is donated by serine. (PMID 16702340)
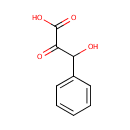
4-Hydroxyphenylpyruvic acid (PAMDB000176)
IUPAC:
3-hydroxy-2-oxo-3-phenylpropanoic acid
CAS: 156-39-8
Description: 4-Hydroxyphenylpyruvic acid (4-HPPA) is a keto acid. It is a product of the enzyme (R)-4-hydroxyphenyllactate dehydrogenase [EC 1.1.1.222] and is formed during tyrosine metabolism (KEGG). There are two isomers of HPPA, specifically 4HPPA and 3HPPA, of which 4HPPA is the most common. The enzyme 4-hydroxyphenylpyruvic acid dioxygenase (HPD) catalyzes the reaction of 4-hydroxyphenylpyruvic acid to homogentisic acid in the tyrosine catabolism pathway.
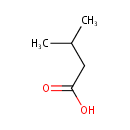
Isovaleric acid (PAMDB000177)
IUPAC:
3-methylbutanoic acid
CAS: 503-74-2
Description: Isovaleric acid, is a natural short-chain fatty acid. It is clear colorless liquid that is sparingly soluble in water, but extremely soluble in most common organic solvents.
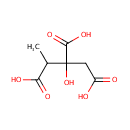
Methylcitric acid (PAMDB000178)
IUPAC:
2-hydroxy-1-methylpropane-1,2,3-tricarboxylic acid
CAS: 6061-96-7
Description: Methylcitric acid (MCA) is formed by condensation of accumulated propionyl- CoA and oxalacetate by the enzyme si-citrate synthase (EC 4.1.3.7). MCA molecule has two stereogenic centers so that it can occur in the form of four stereoisomers.
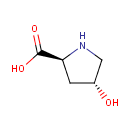
Hydroxyproline (PAMDB000179)
IUPAC:
(2S,4R)-4-hydroxypyrrolidine-2-carboxylic acid
CAS: 51-35-4
Description: Hydroxyproline is a common proteinogenic amino acid. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the C (gamma) atom. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the C (gamma) atom. It is produced by hydroxylation of the amino acid proline. It is not directly coded for by DNA, however, and is hydroxylated after protein synthesis. (Wikipedia)