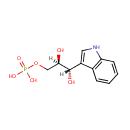Search Results for compounds
Searching compounds for
returned 4373 results.
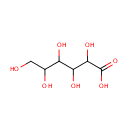
D-Galactonate (PAMDB000988)
IUPAC:
2,3,4,5,6-pentahydroxyhexanoic acid
CAS: 133-42-6
Description: D-galactonate is a member of the chemical class known as Sugar Acids and Derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group. The gluconic acid is produced by direct extracellular oxidation of glucose by a glucose dehydrogenase equipped with pyrroloquinoline quinone (PQQ) as a cofactor. (PMID 14756528) Glucuronate is converted to l-gulonate by aldehyde reductase, an enzyme of the aldo-keto reductase superfamily. l-Gulonate is converted to l-gulonolactone by a lactonase identified as SMP30 or regucalcin, whose absence in mice leads to vitamin C deficiency. (PMID 17222174)
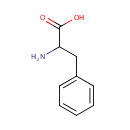
D-Phenylalanine (PAMDB000992)
IUPAC:
2-amino-3-phenylpropanoic acid
CAS: 673-06-3
Description: D-phenylalanine is a member of the chemical class known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). Phenylalanine (abbreviated as Phe or F) is an alpha-amino acid with the formula C6H5CH2CH(NH2)COOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. Metabolic engineering can enhance phenylalanine production in Pseudomonas aeruginosa[PMID: 18080813] D-phenylalanine can be synthesized by glucose fermentation in Pseudomonas aeruginosa using metabolic engineering. DAHP synthase and chorismate mutase-prephenate dehydratase form a pathway where L-phenylalanine synthesis is deregulated and L-phenylalanine is converted into D-phenylalanine [Applied Biocatalysis in Specialty Chemicals and Pharmaceuticals, Chapter 5, p 65-75, Chapter DOI: 10.1021/bk-2001-0776.ch005]
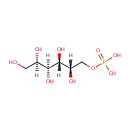
Galactitol 1-phosphate (PAMDB000996)
IUPAC:
{[(2S,3R,4S,5R)-2,3,4,5,6-pentahydroxyhexyl]oxy}phosphonic acid
CAS: 15664-55-8
Description: Galactitol 1-phosphate is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose.
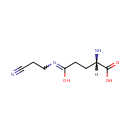
gamma-Glutamyl-beta-aminopropiononitrile (PAMDB000997)
IUPAC:
(2S)-2-amino-4-[(2-cyanoethyl)-C-hydroxycarbonimidoyl]butanoic acid
CAS: Not Available
Description: Gamma-glutamyl-beta-aminopropiononitrile is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon)
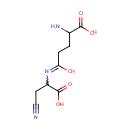
gamma-Glutamyl-beta-cyanoalanine (PAMDB000998)
IUPAC:
2-amino-4-[(1-carboxy-2-cyanoethyl)-C-hydroxycarbonimidoyl]butanoic acid
CAS: Not Available
Description: Gamma-glutamyl-beta-cyanoalanine belongs to the class of Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon).
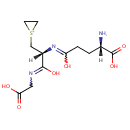
Glutathione episulfonium ion (PAMDB001001)
IUPAC:
1-[(2R)-2-{[(4S)-4-amino-4-carboxy-1-hydroxybutylidene]amino}-2-[(carboxymethyl)-C-hydroxycarbonimidoyl]ethyl]thiiran-1-ium
CAS: Not Available
Description: Glutathione episulfonium ion is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another.
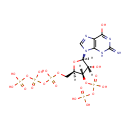
Guanosine 3'-diphosphate 5'-triphosphate (PAMDB001003)
IUPAC:
{[hydroxy({[(2R,3S,4R,5R)-4-hydroxy-2-({[hydroxy({[hydroxy(phosphonooxy)phosphoryl]oxy})phosphoryl]oxy}methyl)-5-(6-hydroxy-2-imino-3,9-dihydro-2H-purin-9-yl)oxolan-3-yl]oxy})phosphoryl]oxy}phosphonic acid
CAS: 38918-96-6
Description: Guanosine 3'-diphosphate 5'-triphosphate is a member of the chemical class known as Purine Ribonucleoside 3',5'-Bisphosphates. These are purine ribobucleotides with one phosphate group attached to 3' and 5' hydroxyl groups of the ribose moiety. The hallmark of the stringent response is the accumulation of guanosine tetra- (ppGpp) and pentaphosphates (pppGpp), which probably bind RNA polymerase to regulate gene expression at certain promoters. (PMID 11282471) Guanosine 5'-triphosphate 3'-diphosphate (pppGpp) and guanosine 5'-diphosphate 3'-diphosphate (ppGpp) are specifically degraded by a manganese-dependent pyrophosphorylase present in spoT+ but not in spoT- strains of Pseudomonas aeruginosa, indicating that the enzyme is the spoT gene product. (PMID 365225) The kinetics of the GTP level during starvation suggests that GTP is a precursor of pppGpp. (PMID 793688)
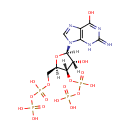
Guanosine 3',5'-bis(diphosphate) (PAMDB001005)
IUPAC:
{[hydroxy({[(2R,3S,4R,5R)-4-hydroxy-2-({[hydroxy(phosphonooxy)phosphoryl]oxy}methyl)-5-(6-hydroxy-2-imino-3,9-dihydro-2H-purin-9-yl)oxolan-3-yl]oxy})phosphoryl]oxy}phosphonic acid
CAS: 33503-72-9
Description: (p)ppGpp, guanosine pentaphosphate or tetraphosphate is an alarmone which is involved in the stringent response in bacteria, causing the inhibition of RNA synthesis when there is a shortage of amino acids present. This causes translation to decrease and the amino acids present are therefore conserved. (p)ppGpp is an effector molecule which is produced as a result of amino acid starvation within a bacterial cell.
Indoleglycerol phosphate (PAMDB001007)
IUPAC:
[(2R,3S)-2,3-dihydroxy-3-(1H-indol-3-yl)propoxy]phosphonic acid
CAS: 4220-97-7
Description: Indoleglycerol phosphate is a member of the chemical class known as Indoles. These are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole. Indoleglycerol phosphate is involved in tryptophan biosynthesis. The latter is the competent substrate of indoleglycerol phosphate synthase, which catalyzes the subsequent step of tryptophan biosynthesis. (PMID 7727401) alphaTS by itself catalyzes the cleavage of indole-3-glycerol phosphate to glyceraldehyde-3-phosphate and indole, which is converted to tryptophan in tryptophan biosynthesis. (PMID 15879705)
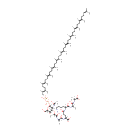
MurAc(oyl-L-Ala-D-gamma-Glu-L-Lys-D-Ala-D-Ala)-diphospho-undecaprenol (PAMDB001020)
IUPAC:
(2R)-4-{[(1S)-5-amino-1-{[(1R)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}pentyl]-C-hydroxycarbonimidoyl}-2-{[(2S)-1-hydroxy-2-[(1-hydroxy-2-{[(2R,3S,4R,5R,6R)-3-hydroxy-6-{[hydroxy({[hydroxy({[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphoryl]oxy})phosphoryl]oxy}-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}propylidene)amino]propylidene]amino}butanoic acid
CAS: Not Available
Description: Murac(oyl-L-ala-D-gamma-glu-L-lys-D-ala-D-ala)-diphospho-undecaprenol is a polyprenol compound involved in the synthesis of peptidoglycan. It is a substrate for the enzyme UDP-N-acetylglucosamine--N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase (murG). This enzyme catalyzes the transfer of a GlcNAc subunit on undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide (lipid intermediate I) to form undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide)GlcNAc (lipid intermediate II). Peptidoglycan is best described as a fisherman net. The mesh of the net is made of two segments of parallel, rather inextensible glycan threads, held together by two small elastic peptide crosslinks allowing the net to expand or shrink. The glycan moiety of the peptidoglycan is remarkably uniform among all bacteria, and is made up of alternating β-1,4-linked N-acetylglucosamine and N-acetyl muramate residues, with an average chain length (in different organisms) of 10 to 65 disaccharide units. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-α-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred by an as yet unknown mechanism through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. Peptide crosslinks form between different parts of the peptides depending on the organism. For example, in Mycobacteria and in Pseudomonas aeruginosa most links form between the carboxyl group of the penultimate D-alanine (residue 4) of one peptide to the amino group at the D-center of meso-diaminopimelate (residue 3) of an adjacent peptide of a second glycan chain (as in Pseudomonas aeruginosa). The crosslinking reaction is catalyzed by transpeptidases and involves the cleavage of the D-alanyl-D-alanine bond of the donor peptide, providing the energy to drive the reaction. As a result, the peptides in the peptidoglycan polymers are one or two amino acids shorter than the peptides in the monomers.