Search Results for compounds
Searching compounds for
returned 4373 results.
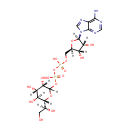
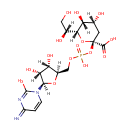
ADP-L-Glycero-D-manno-heptose (PAMDB000961)
IUPAC:
[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]({[(3S,4S,5S,6R)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy})phosphinic acid
CAS: Not Available
Description: ADP-L-glycero-D-manno-heptose is a member of the chemical class known as Purine Nucleotide Sugars. These are purine nucleotides bound to a saccharide derivative through the terminal phosphate group. Functional analysis of the glycero-manno-heptose 7-phosphate kinase domain from the bifunctional HldE protein, which is involved in ADP-L-glycero-D-manno-heptose biosynthesis. (PMID 16030223)
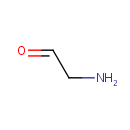
Aminoacetaldehyde (PAMDB000964)
IUPAC:
2-aminoacetaldehyde
CAS: 645-36-3
Description: Aminoacetaldehyde is a member of the chemical class known as Alkylamines. These are organic compounds containing an alkylamine group.
Benzo[a]pyrene-4,5-oxide (PAMDB000966)
IUPAC:
18-oxahexacyclo[10.7.2.0?,??0?????0?????.0??????henicosa-1,3,5,7,9(20),10,12(21),13,15-nonaene
CAS: 37574-47-3
Description: Benzo[a]pyrene-4,5-oxide is a member of the chemical class known as Chrysenes. These are compounds containing the polyaromatic chrysene moiety, which consists of a benzene ring fused to a phenanthrene ring system to form Benzo[a]phenanthrene. In the absence of the ycbX- and yiiM-dependent pathways, biotin sulfoxide reductase plays also a role in the detoxification pathway. (PMID 18312271)
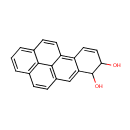
Benzo[a]pyrene-7,8-diol (PAMDB000967)
IUPAC:
pentacyclo[10.6.2.0?,??0?????0??????icosa-1,3,7,9(19),10,12(20),13,15,17-nonaene-5,6-diol
CAS: 13345-25-0
Description: Benzo[a]pyrene-7,8-diol is a member of the chemical class known as Pyrenes. These are compounds containing a pyrene moiety, which consists four fused benzene rings, resulting in a flat aromatic system. Benzo[a]pyrene-7,8-dihydrodiol 9,10-epoxide (BPDE), a metabolite of the widespread environmental pollutant benzo[a]pyrene, is a mutagenic in both bacterial and mammalian systems. (PMID 7890605)
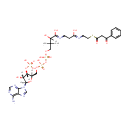
Benzoyl acetyl-CoA (PAMDB000968)
IUPAC:
4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-[2-({2-[(3-oxo-3-phenylpropanoyl)sulfanyl]ethyl}-C-hydroxycarbonimidoyl)ethyl]butanimidic acid
CAS: Not Available
Description: Benzoyl acetyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine.
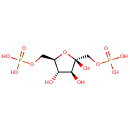
beta-D-Fructose 1,6-bisphosphate (PAMDB000971)
IUPAC:
{[(2R,3S,4S,5R)-2,3,4-trihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methoxy}phosphonic acid
CAS: 125740-83-2
Description: Beta-D-fructose 1,6-bisphosphate is a member of the chemical class known as Pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. The hydrolysis of fructose 1,6-bisphosphate to fructose 6-phosphate is a key reaction of carbohydrate metabolism. (PMID 3008716) Fructose-1,6-bisphosphate (F-1,6-P2) is an allosteric activator of two key enzymes of glycolysis: phosphofructokinase and pyruvate kinase. (PMID 18025560) In gluconeogenesis, fructose 6-phosphate is formed from fructose 1,6-bisphosphate, and if fructose 1,6-bisphosphate were reformed by the phosphofructokinase reaction there would be a "gluconeogenic futile cycle". (PMID 6217196) Fructose-1,6-bisphosphate (FBP) aldolase is an essential glycolytic enzyme that reversibly cleaves its ketohexose substrate into triose phosphates. (PMID 14699122) Fructose 1-phosphate is a metabolite that plays a regulatory role in metabolism and is best measured using an assay based on its conversion to fructose 1,6-bisphosphate by a bacterial fructose-1-phosphate kinase (Fru1PK). (PMID 10833389) For growth, the assimilated fructose is sequentially phosphorylated by ATP and (i) manno(fructo)kinase, to form fructose 6-phosphate, and (ii) phosphofructokinase, to form fructose 1,6-bisphosphate, which is a member of central routes of glycolysis and gluconeogenesis. (PMID 17159144)
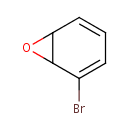
Bromobenzene-2,3-oxide (PAMDB000973)
IUPAC:
2-bromo-7-oxabicyclo[4.1.0]hepta-2,4-diene
CAS: 71942-12-6
Description: Bromobenzene-2,3-oxide is a member of the chemical class known as Organic Compounds. These are compounds containing at least one carbon atom.
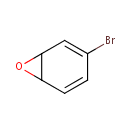
Bromobenzene-3,4-oxide (PAMDB000974)
IUPAC:
3-bromo-7-oxabicyclo[4.1.0]hepta-2,4-diene
CAS: 51981-75-0
Description: Bromobenzene-3,4-oxide is a member of the chemical class known as Organic Compounds. These are compounds containing at least one carbon atom.
CMP-3-Deoxy-D-manno-octulosonate (PAMDB000981)
IUPAC:
(2S,4R,5R,6R)-2-[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(2-hydroxy-4-imino-1,4-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxane-2-carboxylic acid
CAS: Not Available
Description: CMP-3-deoxy-D-manno-octulosonate is a member of the chemical class known as Pyrimidine Nucleotide Sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. CMP KDO is involved in the biosynthesis of lipopolysaccharides. CKS is a pharmaceutical target because CMP-KDO is used in the biosynthesis of lipopolysaccharides that are vital for Gram-negative bacteria. (PMID 11545592) Pseudomonas aeruginosa KdtA (EcKdtA) is a bifunctional enzyme that transfers two KDO units from two CMP-KDO molecules to lipid IV(A). (PMID 20394418)
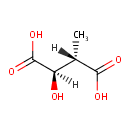
D-Erythro-3-Methylmalate (PAMDB000985)
IUPAC:
(2R,3S)-2-hydroxy-3-methylbutanedioic acid
CAS: Not Available
Description: D-erythro-3-methylmalate is a member of the chemical class known as Dicarboxylic Acids and Derivatives. These are organic compounds containing exactly two carboxylic acid groups.