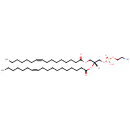Search Results for compounds
Searching compounds for
returned 4373 results.
Potassium (PAMDB000632)
IUPAC:
potassium(1+) ion
CAS: 7440-09-7
Description: Potassium is an essential electrolyte and part of many minerals. Potassium ion is a major intracellular cation in bacteria, plants and animals. It is necessary for the function of all living cells. There are a number of of potassium transport systems that regulate the intracellular potassium concentration.
Pyrophosphate (PAMDB000633)
IUPAC:
(phosphonatooxy)phosphonate
CAS: 14000-31-8
Description: Pyrophosphates are the anions, the salts, and the esters of pyrophosphoric acid. The anion is abbreviated PPi and is formed by the hydrolysis of ATP into AMP in cells. This hydrolysis is called pyrophosphorolysis. The pyrophosphate anion has the structure P2O74-, and is an acid anhydride of phosphate. It is unstable in aqueous solution and rapidly hydrolyzes into inorganic phosphate
PE(16:1(9Z)/16:0) (PAMDB000760)
IUPAC:
(2-aminoethoxy)[(2R)-3-[(9Z)-hexadec-9-enoyloxy]-2-(hexadecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(16:1(9Z)/16:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(16:1(9Z)/16:0), in particular, consists of one 9Z-hexadecenoyl chain to the C-1 atom, and one hexadecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
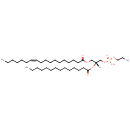
PE(16:1(9Z)/16:1(9Z)) (PAMDB000761)
IUPAC:
(2-aminoethoxy)[(2R)-2,3-bis[(9Z)-hexadec-9-enoyloxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(16:1(9Z)/16:1(9Z)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PE(16:1(9Z)/16:1(9Z)), in particular, consists of two 9Z-hexadecenoyl chains at positions C-1 and C-2. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
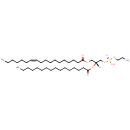
PE(16:1(9Z)/18:1(11Z)) (PAMDB000762)
IUPAC:
(2-aminoethoxy)[(2R)-3-[(9Z)-hexadec-9-enoyloxy]-2-[(11Z)-octadec-11-enoyloxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(16:1(9Z)/18:1(11Z)) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(16:1(9Z)/18:1(11Z)), in particular, consists of one 9Z-hexadecenoyl chain to the C-1 atom, and one 11Z-octadecenoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(18:1(11Z)/14:0) (PAMDB000763)
IUPAC:
(2-aminoethoxy)[(2R)-3-[(11Z)-octadec-11-enoyloxy]-2-(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(18:1(11Z)/14:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(18:1(11Z)/14:0), in particular, consists of one 11Z-octadecenoyl chain to the C-1 atom, and one tetradecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PE(18:1(11Z)/16:0) (PAMDB000764)
IUPAC:
(2-aminoethoxy)[(2R)-2-(hexadecanoyloxy)-3-[(11Z)-octadec-11-enoyloxy]propoxy]phosphinic acid
CAS: Not Available
Description: PE(18:1(11Z)/16:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(18:1(11Z)/16:0), in particular, consists of one 11Z-octadecenoyl chain to the C-1 atom, and one hexadecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
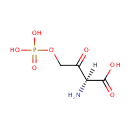
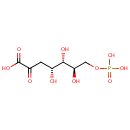
2-Amino-3-oxo-4-phosphonooxybutyrate (PAMDB000885)
IUPAC:
(2S)-2-amino-3-oxo-4-(phosphonooxy)butanoic acid
CAS: Not Available
Description: 2-amino-3-oxo-4-phosphonooxybutyrate is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon)
2-Dehydro-3-deoxy-D-arabino-heptonate 7-phosphate (PAMDB000888)
IUPAC:
(4R,5S,6R)-4,5,6-trihydroxy-2-oxo-7-(phosphonooxy)heptanoic acid
CAS: 2627-73-8
Description: 2-dehydro-3-deoxy-D-arabino-heptonate 7-phosphate is a member of the chemical class known as Medium-chain Keto Acids and Derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. DAHPS is involved in the biosynthesis of aromatic amino acids. Maritima DAHP synthase is a metalloenzyme. This report is the first description of a thermophilic eubacterial DAHP synthase. (PMID 12743122) DAHPS(Phe) is a metal-catalyzed oxidation system wherein bound substrate protects active-site residues from oxidative attack catalyzed by bound redox metal cofactor. (PMID 10049398) The first regulatory step in the synthesis of aromatic amino acids is catalyzed by 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DAHPS). (PMID 15378531) While several similarities exist between the two enzymatic reactions, classic studies on the Pseudomonas aeruginosa enzymes have established that DAHPS is a metalloenzyme, whereas KDO8PS has no metal requirement. aeolicus KDO8PS is a metalloenzyme in vivo and point to a previously unrecognized relationship between the KDO8PS and DAHPS families. (PMID 10811802)
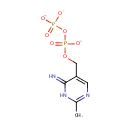
2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate (PAMDB000895)
IUPAC:
{[(6-imino-2-methyl-1,6-dihydropyrimidin-5-yl)methyl phosphonato]oxy}phosphonate
CAS: Not Available
Description: 2-methyl-4-amino-5-hydroxymethylpyrimidine diphosphate is a member of the chemical class known as Organic Pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. HMP-PP is involved in thiamin biosynthesis. Two redundant genes, THI20 and THI21, of Saccharomyces cerevisiae encode a 2-methyl-4-amino-5-hydroxymethylpyrimidine monophosphate (HMP-P) kinase required for thiamin biosynthesis. Although each isoform independently can synthesize HMP pyrophosphate (HMP-PP) from HMP, there is a marked difference in efficiency between the two proteins. (PMID 15614489)