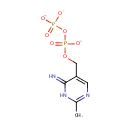
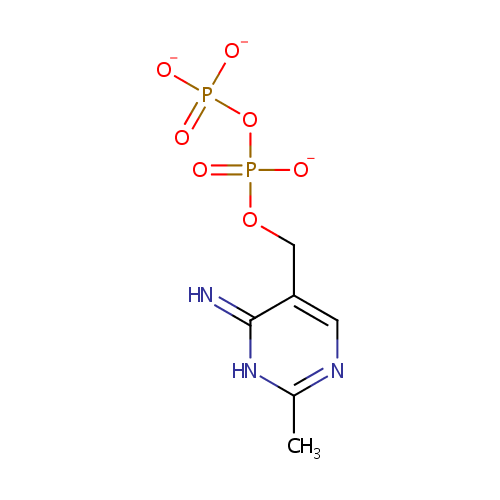
2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate (PAMDB000895)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000895 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2-methyl-4-amino-5-hydroxymethylpyrimidine diphosphate is a member of the chemical class known as Organic Pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. HMP-PP is involved in thiamin biosynthesis. Two redundant genes, THI20 and THI21, of Saccharomyces cerevisiae encode a 2-methyl-4-amino-5-hydroxymethylpyrimidine monophosphate (HMP-P) kinase required for thiamin biosynthesis. Although each isoform independently can synthesize HMP pyrophosphate (HMP-PP) from HMP, there is a marked difference in efficiency between the two proteins. (PMID 15614489) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H8N3O7P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 296.093 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 295.985394345 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | AGQJQCFEPUVXNK-UHFFFAOYSA-K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H11N3O7P2/c1-4-8-2-5(6(7)9-4)3-15-18(13,14)16-17(10,11)12/h2H,3H2,1H3,(H,13,14)(H2,7,8,9)(H2,10,11,12)/p-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(6-imino-2-methyl-1,6-dihydropyrimidin-5-yl)methyl phosphonato]oxy}phosphonate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(4-imino-2-methyl-3H-pyrimidin-5-yl)methyl phosphonato]oxyphosphonate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=NC=C(COP([O-])(=O)OP([O-])([O-])=O)C(=N)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic oxoanionic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Organic pyrophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organic pyrophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + Water > 4-Amino-2-methyl-5-phosphomethylpyrimidine + Hydrogen ion + Phosphate 4-Amino-2-methyl-5-phosphomethylpyrimidine + Adenosine triphosphate > 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + ADP 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole + Hydrogen ion <> Pyrophosphate + Thiamine monophosphate 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + 4-Methyl-5-(2-phosphoethyl)-thiazole <> Pyrophosphate + Thiamine monophosphate 2-[(2<i>R</i>,5<i>Z</i>)-(2-carboxy-4-methylthiazol-5(2<i>H</i>)-ylidene]ethyl phosphate + 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate + Hydrogen ion > Thiamine monophosphate + Carbon dioxide + Pyrophosphate 4-amino-2-methyl-5-diphosphomethylpyrimidine + 2-((2R,5Z)-2-Carboxy-4-methylthiazol-5(2H)-ylidene)ethyl phosphate + 2 Hydrogen ion + 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate > Thiamine monophosphate + Carbon dioxide + diphosphate + Thiamine monophosphate + Pyrophosphate Pyridoxal 5'-phosphate > 4-amino-2-methyl-5-diphosphomethylpyrimidine + 2-Methyl-4-amino-5-hydroxymethylpyrimidine diphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||