Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3651 - 3660 of
4373 in total
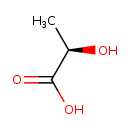
(R)-lactate (PAMDB110741)
IUPAC:
(2R)-2-hydroxypropanoate
CAS: 10326-41-7
Description: An optically active form of lactate having (R)-configuration.
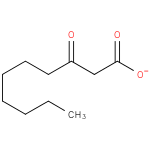
3-oxodecanoate (PAMDB110742)
IUPAC:
3-oxodecanoate
CAS: Not Available
Description: A 3-oxo monocarboxylic acid anion that is the conjugate base of 3-oxodecanoic acid, obtained by deprotonation of the carboxy group.
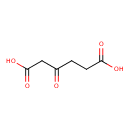
3-oxoadipate (PAMDB110743)
IUPAC:
3-oxohexanedioate
CAS: 689-31-6
Description: A dicarboxylic acid dianion resuting from deprotonation of both carboxy groups of 3-oxoadipic acid.
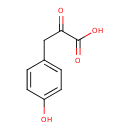
4-hydroxyphenylpyruvate (PAMDB110744)
IUPAC:
3-(4-hydroxyphenyl)-2-oxopropanoate
CAS: 156-39-8
Description: A 2-oxo monocarboxylic acid anion obtained by removal of a proton from the carboxylic acid group of 3-(4-hydroxyphenyl)pyruvic acid.
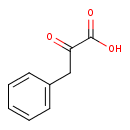
2-oxo-3-phenylpropanoate (PAMDB110745)
IUPAC:
2-oxo-3-phenylpropanoate
CAS: 156-06-9
Description: A 2-oxo monocarboxylic acid anion resulting from deprotonation of the carboxy group of keto-phenylpyruvic acid.
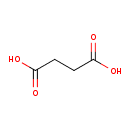
succinate (PAMDB110747)
IUPAC:
butanedioate
CAS: 110-15-6
Description: A dicarboxylic acid dianion resulting from the removal of a proton from both of the carboxy groups of succinic acid.
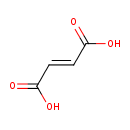
fumarate (PAMDB110748)
IUPAC:
(2E)-but-2-enedioate
CAS: 110-17-8
Description: A C4-dicarboxylate that is the E-isomer of but-2-enedioate(2−)
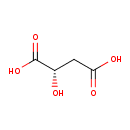
(S)-malate (PAMDB110749)
IUPAC:
(2S)-2-hydroxybutanedioate
CAS: 97-67-6
Description: An optically active form of malate having (S)-configuration.
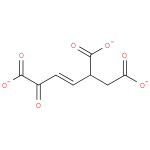
5-carboxy-2-oxohept-3-enedioate (PAMDB110750)
IUPAC:
(3E)-5-oxopent-3-ene-1,2,5-tricarboxylate
CAS: Not Available
Description: Tricarboxylate anion of (3E)-5-oxopent-3-ene-1,2,5-tricarboxylic acid.
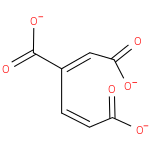
3-carboxy-cis,cis-muconate (PAMDB110751)
IUPAC:
(1E,3Z)-buta-1,3-diene-1,2,4-tricarboxylate
CAS: Not Available
Description: Trianion of 3-carboxy-cis,cis-muconic acid arising from deprotonation of all three carboxylic acid functions.