|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110745 |
|---|
|
Identification |
|---|
| Name: |
2-oxo-3-phenylpropanoate |
|---|
| Description: | A 2-oxo monocarboxylic acid anion resulting from deprotonation of the carboxy group of keto-phenylpyruvic acid. |
|---|
|
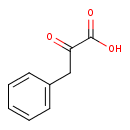
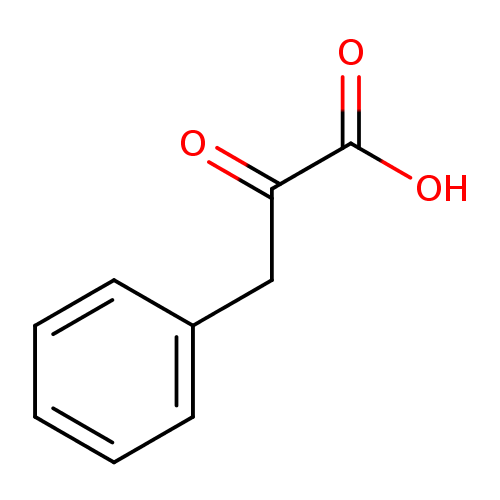
Structure |
|
|---|
| Synonyms: | -
α-ketohydrocinnamic acid
-
3-phenyl-2-oxopropanoate
-
phenylpyruvate
-
3-phenylpyruvate
-
phenylpyruvic acid
|
|---|
|
Chemical Formula: |
C9H7O3
|
|---|
| Average Molecular Weight: |
163.15 |
|---|
| Monoisotopic Molecular
Weight: |
164.0473441231 |
|---|
| InChI Key: |
BTNMPGBKDVTSJY-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C9H8O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,11,12)/p-1 |
|---|
| CAS
number: |
156-06-9 |
|---|
| IUPAC Name: | 2-oxo-3-phenylpropanoate |
|---|
|
Traditional IUPAC Name: |
phenylpyruvic acid |
|---|
| SMILES: | C([O-])(=O)C(=O)CC1(=CC=CC=C1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as phenylpyruvic acid derivatives. These are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
Phenylpyruvic acid derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phenylpyruvate
- 3-phenylpropanoic-acid
- Keto acid
- Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
154 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 154 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 112 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Phenylalanine and Tyrosine Metabolism pae00360
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS) | splash10-00ko-9700000000-1a891469df1de787159b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0006-1900000000-7dc1a5344d13e4dc19ac | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-001i-0900000000-bbbc84c54d03c4a14e51 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0a4i-0900000000-00b547b0371603520b61 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (1 TMS; 1 MEOX) | splash10-00di-9100000000-fdd48b632e63c8478078 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-000f-9300000000-8cd32f87e04ae0b6d6fb | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-014u-9720000000-7a0b66e6c18ff42ac05e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0006-9760000000-d94111a7b9babceb265b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-066r-0900000000-ceb2ce23352e0f45ee33 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-004i-9200000000-ed4a6740d574858da982 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-004i-9000000000-c239a9a599daa607e47f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-00kf-9300000000-d1a7d23e7c5270b16883 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [2026685 ]
- Lee SH, Kim SO, Chung BC: Gas chromatographic-mass spectrometric determination of urinary oxoacids using O-(2,3,4,5,6-pentafluorobenzyl)oxime-trimethylsilyl ester derivatization and cation-exchange chromatography. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):1-7. [9869358 ]
- Boulat O, Gradwohl M, Matos V, Guignard JP, Bachmann C: Organic acids in the second morning urine in a healthy Swiss paediatric population. Clin Chem Lab Med. 2003 Dec;41(12):1642-58. [14708889 ]
- Lasala JM, Coscia CJ: Accumulation of a tetrahydroisoquinoline in phenylketonuria. Science. 1979 Jan 19;203(4377):283-4. [153583 ]
- Cassidei L, Dell'atti A, Sciacovelli O: Improvement of the FeCl3 test for phenylpyruvic acid. Clin Chim Acta. 1978 Dec 1;90(2):121-7. [719897 ]
- Michals K, Matalon R: Phenylalanine metabolites, attention span and hyperactivity. Am J Clin Nutr. 1985 Aug;42(2):361-5. [4025205 ]
- Nakahara T, Ishida J, Yamaguchi M, Nakamura M: Determination of alpha-keto acids including phenylpyruvic acid in human plasma by high-performance liquid chromatography with chemiluminescence detection. Anal Biochem. 1990 Nov 1;190(2):309-13. [2291475 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Synthesis Reference: |
Li, Hongbin; Luo, Yuzhong. Preparation of phenyl-pyruvic acid by dicarbonylation of benzyl halide. Faming Zhuanli Shenqing Gongkai Shuomingshu (1996), 5 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|