Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3571 - 3580 of
4373 in total
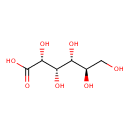
D-gluconate (PAMDB110616)
IUPAC:
D-gluconate
CAS: 526-95-4
Description: A gluconate having D-configuration.
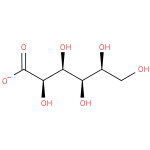
L-idonate (PAMDB110617)
IUPAC:
L-idonate
CAS: Not Available
Description: An optically active form of idonate having L-configuration; major species at pH 7.3.
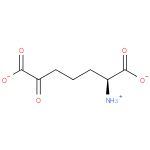
L-α-amino-ε-keto-pimelate (PAMDB110618)
IUPAC:
(2S)-2-ammonio-6-oxoheptanedioate
CAS: Not Available
Description: Conjugate base of (S)-2-amino-6-oxopimelic acid.
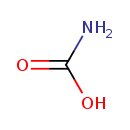
carbamate (PAMDB110619)
IUPAC:
carbamate
CAS: 463-77-4
Description: Carbamic acid is occasionally found as carbamate in workers exposed to pesticides. Carbamates, particularly carbofuran, seem to be more associated with exuberant and diversified symptomatology of pesticide exposure than organophosphates. Neurological symptoms occur among farmers occupationally exposed to acetylcholinesterase-inhibiting insecticides such as carbamates. Carbamic acid products of several amines, such as beta-N-methylamino-L-alanine (BMAA), ethylenediamine, and L-cysteine have been implicated in toxicity. Studies suggested that a significant portion of amino-compounds in biological samples (that naturally contain CO2/bicarbonate) can be present as a carbamic acid. The formation of carbamate glucuronide metabolites has been described for numerous pharmaceuticals and they have been identified in all of the species commonly used in drug metabolism studies (rat, dog, mouse, rabbit, guinea pig, and human). There has been no obvious species specificity for their formation and no preference for 1 or 2 degree amines. Many biological reactions have also been described in the literature that involve the reaction of CO2 with amino groups of biomolecules. For example, CO2 generated from cellular respiration is expired in part through the reversible formation of a carbamate between CO2 and the -amino groups of the alpha and beta-chains of hemoglobin. Glucuronidation is an important mechanism used by mammalian systems to clear and eliminate both endogenous and foreign chemicals. Many functional groups are susceptible to conjugation with glucuronic acid, including hydroxyls, phenols, carboxyls, activated carbons, thiols, amines, and selenium. Primary and secondary amines can also react with carbon dioxide (CO2) via a reversible reaction to form a carbamic acid. The carbamic acid is also a substrate for glucuronidation and results in a stable carbamate glucuronide metabolite. The detection and characterization of these products has been facilitated greatly by the advent of soft ionization mass spectrometry techniques and high field NMR instrumentation. (PMID: 16268118 , 17168688 , 12929145 ).
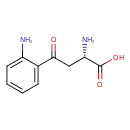
L-kynurenine (PAMDB110620)
IUPAC:
(2S)-4-(2-aminophenyl)-2-azaniumyl-4-oxobutanoate
CAS: 343-65-7
Description: Zwitterionic form of L-kynurenine arising from transfer of a proton from the carboxy to the amino group; major species at pH 7.3.
L-aspartyl-4-phosphate (PAMDB110624)
IUPAC:
(2S)-2-azaniumyl-4-oxo-4-(phosphonatooxy)butanoate
CAS: 22138-53-0
Description: Dianionic form of 4-phosphonato-L-aspartic acid having carboxylic acid and phosphate functions in anionic form and a protonated nitrogen.
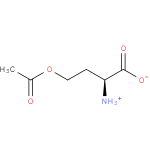
O-acetyl-L-homoserine (PAMDB110625)
IUPAC:
(2S)-4-acetoxy-2-azaniumylbutanoate
CAS: Not Available
Description: Zwitterionic form of O-acetyl-L-homoserine having an anionic carboxy group and a protonated α-amino group; major species at pH 7.3.
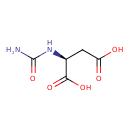
N-carbamoyl-L-aspartate (PAMDB110626)
IUPAC:
N-carbamoyl-L-aspartate
CAS: 13184-27-5
Description: An N-carbamoyl-L-α-amino acid anion obtained by deprotonation of the carboxy groups of N-carbamoyl-L-aspartic acid.
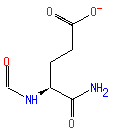
formyl-L-isoglutamine (PAMDB110627)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available