|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110626 |
|---|
|
Identification |
|---|
| Name: |
N-carbamoyl-L-aspartate |
|---|
| Description: | An N-carbamoyl-L-α-amino acid anion obtained by deprotonation of the carboxy groups of N-carbamoyl-L-aspartic acid. |
|---|
|
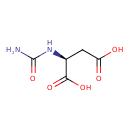
Structure |
|
|---|
| Synonyms: | -
carbamyul-L-aspartate
-
carbamyul-aspartate
-
carbamoyl-aspartate
-
carbamyl-aspartate
-
carbamyl-L-aspartate
|
|---|
|
Chemical Formula: |
C5H6N2O5
|
|---|
| Average Molecular Weight: |
174.11 |
|---|
| Monoisotopic Molecular
Weight: |
176.0433213777 |
|---|
| InChI Key: |
HLKXYZVTANABHZ-REOHCLBHSA-L |
|---|
| InChI: |
InChI=1S/C5H8N2O5/c6-5(12)7-2(4(10)11)1-3(8)9/h2H,1H2,(H,8,9)(H,10,11)(H3,6,7,12)/p-2/t2-/m0/s1 |
|---|
| CAS
number: |
13184-27-5 |
|---|
| IUPAC Name: | N-carbamoyl-L-aspartate |
|---|
|
Traditional IUPAC Name: |
carbamylaspartic acid |
|---|
| SMILES: | C(=O)([O-])CC(NC(N)=O)C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as aspartic acid and derivatives. These are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Aspartic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aspartic acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Isourea
- Carboximidamide
- Carboxylic acid
- Carboximidic acid derivative
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Imine
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
174 - 175 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 174 - 175 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.7 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0f89-3940000000-f6b458a8d8c1bb66550a | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-03di-1910000000-8a8dd39c35aa7c99f81a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-001i-2900000000-8579bc85efea27463b1a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9000000000-bfd715a5dec069d32aea | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-9000000000-17769f0943f627a57c64 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0059-0900000000-d1e136c596eda61fdad6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-001i-1900000000-d9c4b6edb79ec7db500c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-000i-9500000000-8a0f02bbd32fdd0af067 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-000i-9100000000-c1e7be64cc9ef25c1291 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-d90115a5ff29ff776135 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Mehboob S, Mulhearn DC, Truong K, Johnson ME, Santarsiero BD (2010)Structure of dihydroorotase from Bacillus anthracis at 2.6 Å resolution. Acta crystallographica. Section F, Structural biology and crystallization communications 66, Pubmed: 21045288
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|