|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110619 |
|---|
|
Identification |
|---|
| Name: |
carbamate |
|---|
| Description: | Carbamic acid is occasionally found as carbamate in workers exposed to pesticides. Carbamates, particularly carbofuran, seem to be more associated with exuberant and diversified symptomatology of pesticide exposure than organophosphates. Neurological symptoms occur among farmers occupationally exposed to acetylcholinesterase-inhibiting insecticides such as carbamates. Carbamic acid products of several amines, such as beta-N-methylamino-L-alanine (BMAA), ethylenediamine, and L-cysteine have been implicated in toxicity. Studies suggested that a significant portion of amino-compounds in biological samples (that naturally contain CO2/bicarbonate) can be present as a carbamic acid. The formation of carbamate glucuronide metabolites has been described for numerous pharmaceuticals and they have been identified in all of the species commonly used in drug metabolism studies (rat, dog, mouse, rabbit, guinea pig, and human). There has been no obvious species specificity for their formation and no preference for 1 or 2 degree amines. Many biological reactions have also been described in the literature that involve the reaction of CO2 with amino groups of biomolecules. For example, CO2 generated from cellular respiration is expired in part through the reversible formation of a carbamate between CO2 and the -amino groups of the alpha and beta-chains of hemoglobin. Glucuronidation is an important mechanism used by mammalian systems to clear and eliminate both endogenous and foreign chemicals. Many functional groups are susceptible to conjugation with glucuronic acid, including hydroxyls, phenols, carboxyls, activated carbons, thiols, amines, and selenium. Primary and secondary amines can also react with carbon dioxide (CO2) via a reversible reaction to form a carbamic acid. The carbamic acid is also a substrate for glucuronidation and results in a stable carbamate glucuronide metabolite. The detection and characterization of these products has been facilitated greatly by the advent of soft ionization mass spectrometry techniques and high field NMR instrumentation. (PMID: 16268118 , 17168688 , 12929145 ). |
|---|
|
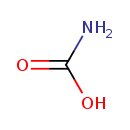
Structure |
|
|---|
| Synonyms: | -
carbamic acid
-
aminoformic acid
|
|---|
|
Chemical Formula: |
CH2NO2
|
|---|
| Average Molecular Weight: |
60.032 |
|---|
| Monoisotopic Molecular
Weight: |
61.0163783457 |
|---|
| InChI Key: |
KXDHJXZQYSOELW-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/CH3NO2/c2-1(3)4/h2H2,(H,3,4)/p-1 |
|---|
| CAS
number: |
463-77-4 |
|---|
| IUPAC Name: | carbamate |
|---|
|
Traditional IUPAC Name: |
carbamic acid |
|---|
| SMILES: | C(=O)([O-])N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as carbamic acids. These are compounds containing the carbamic acid functional group, with the general structure RN(R')C(=O)OH or RN(R')C(=O)[O-] where R,R' = H or organyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Carbamic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Carbamic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Rendon von Osten J, Epomex C, Tinoco-Ojanguren R, Soares AM, Guilhermino L: Effect of pesticide exposure on acetylcholinesterase activity in subsistence farmers from Campeche, Mexico. Arch Environ Health. 2004 Aug;59(8):418-25. [16268118 ]
- Schaefer WH: Reaction of primary and secondary amines to form carbamic acid glucuronides. Curr Drug Metab. 2006 Dec;7(8):873-81. [17168688 ]
- Smit LA, van-Wendel-de-Joode BN, Heederik D, Peiris-John RJ, van der Hoek W: Neurological symptoms among Sri Lankan farmers occupationally exposed to acetylcholinesterase-inhibiting insecticides. Am J Ind Med. 2003 Sep;44(3):254-64. [12929145 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|