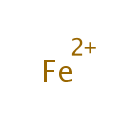Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 2321 - 2330 of
4373 in total
Fe2+ (PAMDB005697)
IUPAC:
??-iron(2+) ion
CAS: Not Available
Description: Iron is a chemical element with the symbol Fe and atomic number 26. Iron makes up 5% of the Earth's crust and is second in abundance to aluminium among the metals and fourth in abundance among the elements. Physiologically, it. exists as an ion in the body. Iron (as Fe2+, ferrous ion) is a necessary trace element used by all known living organisms. Iron-containing enzymes, usually containing heme prosthetic groups, participate in catalysis of oxidation reactions in biology, and in transport of a number of soluble gases. Iron is an essential constituent of hemoglobin, cytochrome, and other components of respiratory enzyme systems. Its chief functions are in the transport of oxygen to tissue (hemoglobin) and in cellular oxidation mechanisms. Inorganic iron involved in redox reactions is also found in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. A class of non-heme iron proteins is responsible for a wide range of functions such as ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis) and purple acid phosphatase (hydrolysis of phosphate esters). When the body is fighting a bacterial infection, the body sequesters iron inside of cells (mostly stored in the storage molecule ferritin) so that it cannot be used by bacteria. Depletion of iron stores may result in iron-deficiency anemia. Iron is used to build up the blood in anemia. Humans experience iron toxicity above 20 milligrams of iron for every kilogram of weight, and 60 milligrams per kilogram is a lethal dose. Over-consumption of iron, often the result of children eating large quantities of ferrous sulfate tablets intended for adult consumption, is the most common toxicological cause of death in children under six. The DRI lists the Tolerable Upper Intake Level (UL) for adults as 45 mg/day. For children under fourteen years old the UL is 40 mg/day. Iron is a metal extracted from iron ore, and is almost never found in the free elemental state.
5,10-Methenyltetrahydrofolic acid (PAMDB006184)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
D-threo-Isocitric acid (PAMDB006191)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
5'-phosphoribosyl-a-N-formylglycineamidine (PAMDB006195)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine (PAMDB006206)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
DG(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:1(11Z)/0:0) (PAMDB006215)
IUPAC:
(2R)-2-hydroxy-3-[(11Z)-octadec-11-enoyloxy]propyl (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoate
CAS: Not Available
Description: DG(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:1(11Z)/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:1(11Z)/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway.