| Identification |
| Name: |
Methionyl-tRNA formyltransferase |
| Synonyms: |
Not Available |
| Gene Name: |
fmt |
| Enzyme Class: |
|
| Biological Properties |
| General Function: |
Involved in methionyl-tRNA formyltransferase activity |
| Specific Function: |
Modifies the free amino group of the aminoacyl moiety of methionyl-tRNA(fMet). The formyl group appears to play a dual role in the initiator identity of N-formylmethionyl-tRNA by:(I) promoting its recognition by IF2 and (II) impairing its binding to EFTu-GTP |
| Cellular Location: |
Not Available |
| KEGG Pathways: |
|
| KEGG Reactions: |
|
L-Methionyl-tRNA | + | 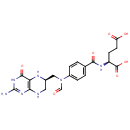 | ↔ | 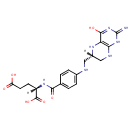 | + | N-Formylmethionyl-tRNA |
| |
|
| SMPDB Reactions: |
|
Tetrahydrofolic acid | + | N-formylmethionyl-tRNA(fMet) | + |  | ? | L-methionyl-tRNA(Met) | + | 10-Formyltetrahydrofolate | + |  |
| |
|
| PseudoCyc/BioCyc Reactions: |
|
| Complex Reactions: |
|
 | + | L-Methionyl-tRNA (Met) | → | N-Formylmethionyl-tRNA | + |  | + |  |
| | |
 | + | L-methionyl-tRNA(fMet) | → |  | + | N-formylmethionyl-tRNA(fMet) |
| |
|
| Transports: |
Not Available |
| Metabolites: |
|
| GO Classification: |
| Function |
|---|
| catalytic activity | | glycine hydroxymethyltransferase activity | | hydroxymethyl-, formyl- and related transferase activity | | methionyl-tRNA formyltransferase activity | | methyltransferase activity | | transferase activity | | transferase activity, transferring one-carbon groups | | Process |
|---|
| biosynthetic process | | cellular macromolecule biosynthetic process | | macromolecule biosynthetic process | | metabolic process | | translation |
|
| Gene Properties |
| Locus tag: |
PA0018 |
| Strand: |
- |
| Entrez Gene ID: |
879258 |
| Accession: |
NP_248708.1 |
| GI: |
15595216 |
| Sequence start: |
20068 |
| Sequence End: |
21012 |
| Sequence Length: |
944 |
| Gene Sequence: |
>PA0018
ATGAGCCAAGCATTGCGCATCGTCTTCGCCGGAACCCCGGAATTCGCCGCCGAGCATCTCAAGGCCCTGCTCGACACCCCACATCGGATCGTCGCCGTCTACACCCAGCCTGACCGGCCGGCCGGCCGCGGGCAGAAACTGATGCCCAGCGCGGTGAAGAGCCTGGCCCTGGAGCATGGCCTGCCGGTCATGCAGCCGCAGAGCCTGCGTAATGCCGAGGCCCAGGCGGAGCTGGCGGCCCTGCGCGCGGACCTGATGGTGGTGGTCGCCTATGGCCTGATCCTGCCCCAGGCGGTACTCGATATCCCGCGCCTGGGCTGCATCAACAGCCACGCCTCGCTGCTGCCGCGCTGGCGCGGCGCCGCGCCGATCCAGCGCGCGGTGGAAGCCGGCGACGCGGAGAGCGGCGTCACCGTGATGCAGATGGAAGCAGGGCTCGACACCGGCCCGATGCTGCTCAAGGTGAGCACGCCGATTTCCGCCGCGGACACCGGCGGCAGCCTGCACGATCGGCTCGCCGCGCTCGGCCCGAAAGCGGTGATCGAAGCCATCGCCGGCCTGGCCGCCGGCACCCTGCATGGCGAGATCCAGGACGACGCCCTGGCCACCTACGCGCACAAGCTGAACAAGGACGAGGCACGCCTCGACTGGAGCCGTCCGGCCGTCGAACTGGAGCGCCAGGTCCGCGCCTTCACCCCCTGGCCGGTCTGCCACACCAGCCTCGCCGATGCGCCGCTGAAAGTCCTCGGCGCCAGCCTGGGGCAGGGCAGCGGGGCGCCCGGAACCATCCTCGAGGCCAGCCGCGACGGCCTGCTGGTCGCCTGCGGCGAAGGCGCCCTGCGCCTGACCCGCCTGCAATTGCCTGGCGGCAAGCCACTGGCCTTCGCCGACCTCTACAACAGCCGCCGCGAGCAATTCGCCGCCGGCCAGGTGCTCGGCCAATGA |
| Protein Properties |
| Protein Residues: |
314 |
| Protein Molecular Weight: |
33 kDa |
| Protein Theoretical pI: |
6.96 |
| Hydropathicity (GRAVY score): |
0.068 |
| Charge at pH 7 (predicted): |
-0.17 |
| Protein Sequence: |
>PA0018
MSQALRIVFAGTPEFAAEHLKALLDTPHRIVAVYTQPDRPAGRGQKLMPSAVKSLALEHGLPVMQPQSLRNAEAQAELAALRADLMVVVAYGLILPQAVLDIPRLGCINSHASLLPRWRGAAPIQRAVEAGDAESGVTVMQMEAGLDTGPMLLKVSTPISAADTGGSLHDRLAALGPKAVIEAIAGLAAGTLHGEIQDDALATYAHKLNKDEARLDWSRPAVELERQVRAFTPWPVCHTSLADAPLKVLGASLGQGSGAPGTILEASRDGLLVACGEGALRLTRLQLPGGKPLAFADLYNSRREQFAAGQVLGQ |
| References |
| External Links: |
|
| General Reference: |
PaperBLAST - Find papers about PA0018 and its homologs
|