|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110387 |
|---|
|
Identification |
|---|
| Name: |
malonyl-CoA |
|---|
| Description: | Pentaanion of malonyl-CoA arising from deprotonation of phosphate, diphosphate and carboxylic acid functions. |
|---|
|
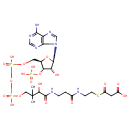
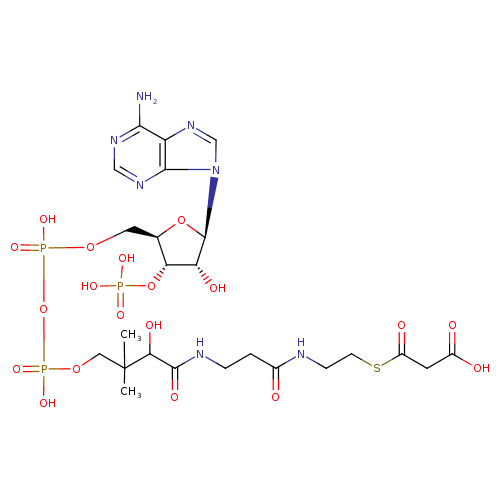
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C24H33N7O19P3S
|
|---|
| Average Molecular Weight: |
848.54 |
|---|
| Monoisotopic Molecular
Weight: |
853.1156022961 |
|---|
| InChI Key: |
LTYOQGRJFJAKNA-DVVLENMVSA-I |
|---|
| InChI: |
InChI=1S/C24H38N7O19P3S/c1-24(2,19(37)22(38)27-4-3-13(32)26-5-6-54-15(35)7-14(33)34)9-47-53(44,45)50-52(42,43)46-8-12-18(49-51(39,40)41)17(36)23(48-12)31-11-30-16-20(25)28-10-29-21(16)31/h10-12,17-19,23,36-37H,3-9H2,1-2H3,(H,26,32)(H,27,38)(H,33,34)(H,42,43)(H,44,45)(H2,25,28,29)(H2,39,40,41)/p-5/t12-,17-,18-,19+,23-/m1/s1 |
|---|
| CAS
number: |
524-14-1 |
|---|
| IUPAC Name: | 3'- phosphonatoadenosine 5'- phosphonatoadenosine 5'- {3- {3- [(3R)- [(3R)- 4- 4- {[3- {[3- ({2- ({2- [(3- [(3- carboxylatoacetyl)sulfanyl]ethyl}amino)- carboxylatoacetyl)sulfanyl]ethyl}amino)- 3- 3- oxopropyl]amino}- oxopropyl]amino}- 3- 3- hydroxy- hydroxy- 2,2- 2,2- dimethyl- dimethyl- 4- 4- oxobutyl] diphosphate} oxobutyl] diphosphate} |
|---|
|
Traditional IUPAC Name: |
3-({2-[3-(3-{[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-2-hydroxy-3-methylbutanamido)propanamido]ethyl}sulfanyl)-3-oxopropanoic acid |
|---|
| SMILES: | CC(C)(C(O)C(=O)NCCC(=O)NCCSC(=O)CC(=O)[O-])COP(=O)(OP(=O)(OCC1(C(OP([O-])(=O)[O-])C(O)C(O1)N3(C2(=C(C(N)=NC=N2)N=C3))))[O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Ribonucleoside 3'-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Fatty amide
- Imidolactam
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Alkyl phosphate
- 1,3-dicarbonyl compound
- Phosphoric acid ester
- Primary aromatic amine
- Pyrimidine
- Oxolane
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Carbothioic s-ester
- Amino acid
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic nitrogen compound
- Alcohol
- Amine
- Organonitrogen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -5 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B: Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005 Jan;288(1):E133-42. Epub 2004 Sep 21. [15383373 ]
- Napal L, Dai J, Treber M, Haro D, Marrero PF, Woldegiorgis G: A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of rat liver carnitine palmitoyltransferase I increases its malonyl-CoA sensitivity close to that observed with the muscle isoform of the enzyme. J Biol Chem. 2003 Sep 5;278(36):34084-9. Epub 2003 Jun 25. [12826662 ]
- Kuhl JE, Ruderman NB, Musi N, Goodyear LJ, Patti ME, Crunkhorn S, Dronamraju D, Thorell A, Nygren J, Ljungkvist O, Degerblad M, Stahle A, Brismar TB, Andersen KL, Saha AK, Efendic S, Bavenholm PN: Exercise training decreases the concentration of malonyl-CoA and increases the expression and activity of malonyl-CoA decarboxylase in human muscle. Am J Physiol Endocrinol Metab. 2006 Jun;290(6):E1296-303. Epub 2006 Jan 24. [16434556 ]
- Odland LM, Howlett RA, Heigenhauser GJ, Hultman E, Spriet LL: Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol. 1998 Jun;274(6 Pt 1):E1080-5. [9611159 ]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM: Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006 Aug;55(8):2277-85. [16873691 ]
- Pender C, Trentadue AR, Pories WJ, Dohm GL, Houmard JA, Youngren JF: Expression of genes regulating malonyl-CoA in human skeletal muscle. J Cell Biochem. 2006 Oct 15;99(3):860-7. [16721829 ]
- Prentki M, Corkey BE: Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996 Mar;45(3):273-83. [8593930 ]
- Bavenholm PN, Pigon J, Saha AK, Ruderman NB, Efendic S: Fatty acid oxidation and the regulation of malonyl-CoA in human muscle. Diabetes. 2000 Jul;49(7):1078-83. [10909961 ]
- Ruderman NB, Saha AK, Vavvas D, Witters LA: Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol. 1999 Jan;276(1 Pt 1):E1-E18. [9886945 ]
- Saha AK, Ruderman NB: Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol Cell Biochem. 2003 Nov;253(1-2):65-70. [14619957 ]
- Morillas M, Gomez-Puertas P, Bentebibel A, Selles E, Casals N, Valencia A, Hegardt FG, Asins G, Serra D: Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for malonyl-CoA inhibition. Mutation of methionine 593 abolishes malonyl-CoA inhibition. J Biol Chem. 2003 Mar 14;278(11):9058-63. Epub 2002 Dec 23. [12499375 ]
- Odland LM, Heigenhauser GJ, Lopaschuk GD, Spriet LL: Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol. 1996 Mar;270(3 Pt 1):E541-4. [8638703 ]
- Trevisan CP, Angelini C, Fiorellini LA, Isaya G, Zacchello G: Malonyl-CoA abnormal inhibition of residual enzyme activity in carnitine palmitoyltransferase deficiency. Eur Neurol. 1986;25(4):309-16. [3720808 ]
|
|---|
| Synthesis Reference: |
Hulsmann, W. C. Synthesis of malonyl coenzyme A from acetyl coenzyme A and oxalosuccinate in mitochondria. Biochimica et Biophysica Acta (1963), 77(3), 502-3. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|