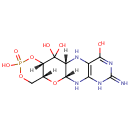
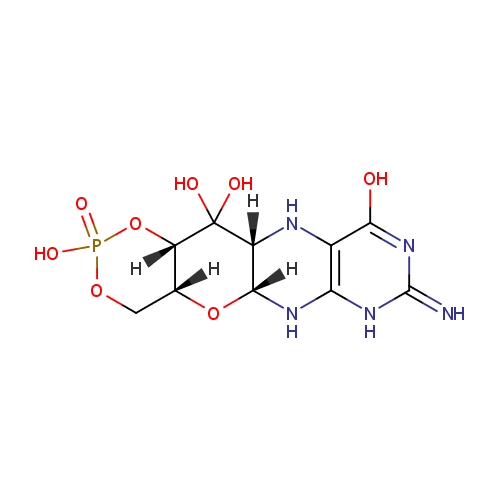
Cyclic pyranopterin monophosphate (PAMDB001401)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001401 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Cyclic pyranopterin monophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Cyclic pyranopterin monophosphate is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. cPMP is a precursor to molybdenum cofactor, which is required for the enyzme activity of sulfite oxidase, xanthine dehydrogenase/oxidase and aldehyde oxidase. The transition element molybdenum (Mo) has been long known as an essential micronutrient across the kingdoms of plants, animals, fungi and bacteria. However, molybdate itself is catalytically inactive and, with the exception of bacterial nitrogenase, needs to be activated through complexation by a special cofactor. There are several molybdenum cofactors, including molybdopterin (MPT), guanylyl molybdenum cofactor (MGD), cytidylyl molybdenum cofactor, or others. In Pseudomonas aeruginosa, the MoaD protein plays a central role in the conversion of precursor Z to molybdopterin (MPT) during molybdenum cofactor biosynthesis. (PMID 17223713) In Pseudomonas aeruginosa, MPT is formed by incorporation of two sulfur atoms into precursor Z, which is catalyzed by MPT synthase. (PMID 11459846) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C10H14N5O8P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 363.2206 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 363.057998961 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | CZAKJJUNKNPTTO-AJFJRRQVSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C10H14N5O8P/c11-9-14-6-3(7(16)15-9)12-4-8(13-6)22-2-1-21-24(19,20)23-5(2)10(4,17)18/h2,4-5,8,12,17-18H,1H2,(H,19,20)(H4,11,13,14,15,16)/t2-,4-,5+,8-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (4aR,5aR,11aR,12aS)-2,10,12,12-tetrahydroxy-8-imino-4,4a,5a,6,7,8,11,11a,12,12a-decahydro-2H-1,3,5-trioxa-6,7,9,11-tetraaza-2???phosphatetracen-2-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (4aR,5aR,11aR,12aS)-2,10,12,12-tetrahydroxy-8-imino-4,4a,5a,6,7,11,11a,12a-octahydro-1,3,5-trioxa-6,7,9,11-tetraaza-2???phosphatetracen-2-one | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@@]12COP(O)(=O)O[C@]1([H])C(O)(O)[C@]1([H])NC3=C(NC(=N)N=C3O)N[C@]1([H])O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyranopterins and derivatives. These are pterin derivatives in which a pyran ring is fused either to the pyrimidine ring or the pyrazine ring of the pterin moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pteridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pterins and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyranopterins and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Guanosine triphosphate + Water <> Cyclic pyranopterin monophosphate + Pyrophosphate Cyclic pyranopterin monophosphate + Copper + 2 MoaD Protein with thiocarboxylate >5 Hydrogen ion +2 MoaD Protein with carboxylate + Molybdopterin Cyclic pyranopterin monophosphate + 2 Sulfur donor <> Molybdopterin Guanosine triphosphate > Cyclic pyranopterin monophosphate + Pyrophosphate Cyclic pyranopterin monophosphate + Water + Thiocarboxylated-MPT-synthases > Molybdopterin + MPT-Synthases Guanosine triphosphate > Cyclic pyranopterin monophosphate + Pyrophosphate Cyclic pyranopterin monophosphate + Water + thiocarboxylated small subunit of molybdopterin synthase >4 Hydrogen ion +2 thiocarboxylated small subunit of molybdopterin synthase + Molybdopterin + Molybdopterin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in Mo-molybdopterin cofactor biosynthetic process
- Specific function:
- Involved in sulfur transfer in the conversion of molybdopterin precursor Z to molybdopterin
- Gene Name:
- moaD
- Locus Tag:
- PA3917
- Molecular weight:
- 9.2 kDa
- General function:
- Involved in Mo-molybdopterin cofactor biosynthetic process
- Specific function:
- Converts molybdopterin precursor Z to molybdopterin. This requires the incorporation of two sulfur atoms into precursor Z to generate a dithiolene group. The sulfur is provided by moaD
- Gene Name:
- moaE
- Locus Tag:
- PA3916
- Molecular weight:
- 16.7 kDa
Reactions
| Cyclic pyranopterin monophosphate + 2 [molybdopterin-synthase sulfur-carrier protein]-Gly-NH-CH(2)-C(O)SH + H(2)O = molybdopterin + 2 [molybdopterin-synthase sulfur-carrier protein]. |
- General function:
- Involved in Mo-molybdopterin cofactor biosynthetic process
- Specific function:
- Together with moaA, is involved in the conversion of a guanosine derivative (5'-GTP) into molybdopterin precursor Z
- Gene Name:
- moaC
- Locus Tag:
- PA3918
- Molecular weight:
- 17.3 kDa