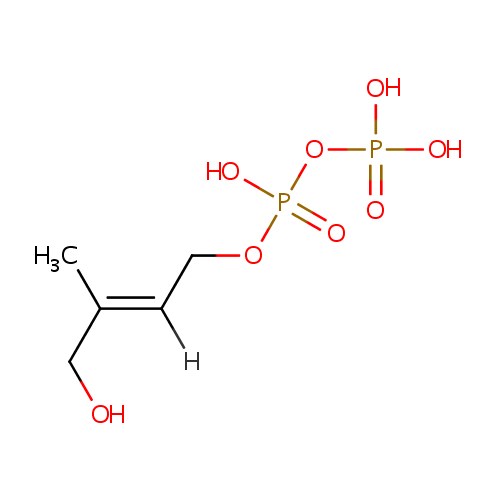
1-Hydroxy-2-methyl-2-butenyl 4-diphosphate (PAMDB000869)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000869 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 1-hydroxy-2-methyl-2-butenyl 4-diphosphate is a member of the chemical class known as Organic Pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H12O8P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 262.0915 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 262.000740384 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | MDSIZRKJVDMQOQ-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H12O8P2/c1-5(4-6)2-3-12-15(10,11)13-14(7,8)9/h2,6H,3-4H2,1H3,(H,10,11)(H2,7,8,9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[hydroxy({[(2E)-4-hydroxy-3-methylbut-2-en-1-yl]oxy})phosphoryl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | {hydroxy[(2E)-4-hydroxy-3-methylbut-2-en-1-yl]oxyphosphoryl}oxyphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(CO)=CCOP(O)(=O)OP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-C-Methyl-D-erythritol-2,4-cyclodiphosphate + 2 Flavodoxin reduced + Hydrogen ion >2 flavodoxin semi oxidized + 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + Water Hydrogen ion + 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADH > Water + Isopentenyl pyrophosphate + NAD Hydrogen ion + 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADH > Dimethylallylpyrophosphate + Water + NAD 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADPH + Hydrogen ion <> Isopentenyl pyrophosphate + NADP + Water Dimethylallylpyrophosphate + NADP + Water <> 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADPH + Hydrogen ion 2-C-Methyl-D-erythritol-2,4-cyclodiphosphate + 2 Reduced ferredoxin + Oxidized ferredoxin <> 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + Water +2 Oxidized ferredoxin + Reduced ferredoxin Isopentenyl pyrophosphate + NAD(P)<sup>+</sup> + Water < 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NAD(P)H + Hydrogen ion 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + Water + Oxidized-ferredoxins < 2-C-Methyl-D-erythritol-2,4-cyclodiphosphate + Hydrogen ion + Reduced-ferredoxins NAD(P)<sup>+</sup> + Dimethylallylpyrophosphate + Water < NAD(P)H + Hydrogen ion + 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + Water + 2 oxidized ferredoxin > 2-C-Methyl-D-erythritol 2,4-cyclodiphosphate +2 reduced ferredoxin Isopentenyl pyrophosphate + NAD(P)(+) + Water > 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NAD(P)H Dimethylallylpyrophosphate + NAD(P)(+) + Water > 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NAD(P)H Isopentenyl pyrophosphate + NAD + NADP + Water + Dimethylallylpyrophosphate <> 1-Hydroxy-2-methyl-2-butenyl 4-diphosphate + NADH + NADPH + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Converts 2C-methyl-D-erythritol 2,4-cyclodiphosphate (ME-2,4cPP) into 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate
- Gene Name:
- ispG
- Locus Tag:
- PA3803
- Molecular weight:
- 40.1 kDa
Reactions
| (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate + H(2)O + 2 oxidized ferredoxin = 2-C-methyl-D-erythritol 2,4-cyclodiphosphate + 2 reduced ferredoxin. |
- General function:
- Involved in isopentenyl diphosphate biosynthetic process, mevalonate-independent pathway
- Specific function:
- Converts 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Is also involved in penicillin tolerance and control of the stringent response. Seems to directly or indirectly interact with relA to maintain it in an inactive form during normal growth
- Gene Name:
- ispH
- Locus Tag:
- PA4557
- Molecular weight:
- 34.8 kDa
Reactions
| Isopentenyl diphosphate + NAD(P)(+) + H(2)O = (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate + NAD(P)H. |
| Dimethylallyl diphosphate + NAD(P)(+) + H(2)O = (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate + NAD(P)H. |