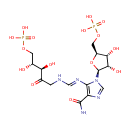
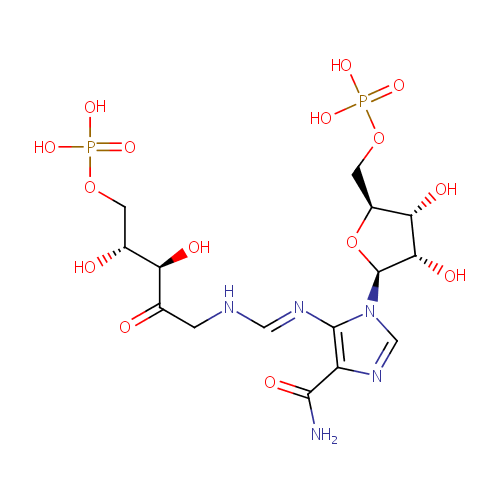
Phosphoribulosylformimino-AICAR-P (PAMDB000838)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000838 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Phosphoribulosylformimino-AICAR-P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Phosphoribulosylformimino-aicar-p is a member of the chemical class known as 1-Phosphoribosyl-imidazolecarboxamides. These are organic compounds containing the imidazolecarboxamide linked to a ribose phosphate through a 1-2 bond. PRFAR is catalyzed by imidazole glycerol phosphate syntahse. Imidazole glycerol phosphate (IGP) synthase is a glutamine amidotransferase that catalyzes the formation of IGP and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) from N(1)-[(5'-phosphoribulosyl)formimino]-5-aminoimidazole-4-car boxamide ribonucleotide (PRFAR). (PMID 10733892). 5-((5-phospho-1-deoxyribulos-1-ylamino)methylideneamino)-1-(5-phosphoribosyl)imidazole-4-carboxamide is an intermediate in the histidine biosynthesis pathway. It is a substrate for the enzyme 1-(5-phosphoribosyl)-5-[(5-phosphoribosylamino)methylideneamino] imidazole-4-carboxamide isomerase which catalyzes the reaction 1-(5-phosphoribosyl)-5-((5-phosphoribosylamino)methylideneamino)imidazole-4-carboxamide = 5-((5-phospho-1-deoxyribulos-1-ylamino)methylideneamino)-1-(5-phosphoribosyl)imidazole-4-carboxamide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C15H25N5O15P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 577.331 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 577.082238179 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | BLKFNHOCHNCLII-IIZOACFYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C15H25N5O15P2/c16-13(26)9-14(18-4-17-1-6(21)10(23)7(22)2-33-36(27,28)29)20(5-19-9)15-12(25)11(24)8(35-15)3-34-37(30,31)32/h4-5,7-8,10-12,15,22-25H,1-3H2,(H2,16,26)(H,17,18)(H2,27,28,29)(H2,30,31,32)/t7-,8+,10+,11+,12+,15+/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3R)-5-[(E)-N'-{4-carbamoyl-1-[(2S,3S,4R,5S)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-1H-imidazol-5-yl}methenimidamido]-2,3-dihydroxy-4-oxopentyl]oxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(2R,3R)-5-[(E)-N'-{5-carbamoyl-3-[(2S,3S,4R,5S)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]imidazol-4-yl}methenimidamido]-2,3-dihydroxy-4-oxopentyl]oxyphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H](COP(=O)(O)O)[C@@H](O)C(=O)CN\C=N\C1=C(C(=O)N)N=CN1[C@H]1O[C@@H](COP(=O)(O)O)[C@H](O)[C@@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as 1-phosphoribosyl-imidazolecarboxamides. These are organic compounds containing the imidazolecarboxamide linked to a ribose phosphate through a 1-2 bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Imidazole ribonucleosides and ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1-ribosyl-imidazolecarboxamides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1-phosphoribosyl-imidazolecarboxamides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Glutamine + Phosphoribulosylformimino-AICAR-P > Phosphoribosyl formamidocarboxamide + D-Erythro-imidazole-glycerol-phosphate + L-Glutamate + Hydrogen ion Phosphoribulosylformimino-AICAR-P + L-Glutamine <> D-Erythro-imidazole-glycerol-phosphate + AICAR + L-Glutamate PhosphoribosylformiminoAICAR-phosphate <> Phosphoribulosylformimino-AICAR-P Phosphoribulosylformimino-AICAR-P + L-Glutamine > Hydrogen ion + L-Glutamate + D-Erythro-imidazole-glycerol-phosphate + AICAR Phosphoribulosylformimino-AICAR-P + L-Glutamine > L-Glutamic acid + Hydrogen ion + 5-Amino-4-imidazolecarboxyamide + D-Erythro-imidazole-glycerol-phosphate + L-Glutamate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||