|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000667 |
|---|
|
Identification |
|---|
| Name: |
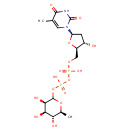
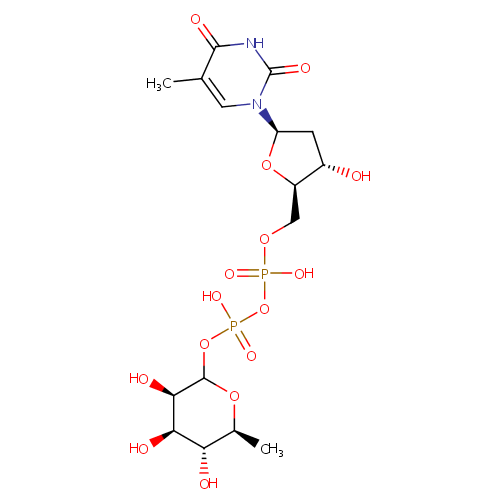
Deoxythymidine diphosphate-L-rhamnose |
|---|
| Description: | Deoxythymidine diphosphate (dTDP)-L-rhamnose is the precursor of L-rhamnose, a saccharide required for the virulence of some pathogenic bacteria. In gram negative bacteria such as Salmonella enterica, Vibrio cholerae or Pseudomonas aeruginosa 075:K5, L-rhamnose is an important residue in the O-antigen of lipopolysaccharides, which are essential for resistance to serum killing and for colonization. dTDP-L-rhamnose is synthesized from glucose-1-phosphate and deoxythymidine triphosphate (dTTP) via a pathway involving four distinct enzymes. Whereas common sugars such as glucose, fructose and mannose are all D-configured, bacteria commonly utilize the L-configured carbohydrates in pharmacologically active compounds and in their cell-wall structures. The enzymes involved in dTDP-L-rhamnose synthesis are potential targets for the design of new therapeutic agents against bacteria. (PMID 10802738, 12773151) |
|---|
|
Structure |
|
|---|
| Synonyms: | - Deoxythymidine diphosphoric acid-L-rhamnose
- DTDP-6-Deoxy-L-mannose
- DTDP-L-Rhamnose
- DTDP-Rhamnose
- TDP-Rhamnose
- Thymidine diphosphate rhamnose
- Thymidine diphosphate-L-rhamnose
- Thymidine diphosphoric acid rhamnose
- Thymidine diphosphoric acid-L-rhamnose
|
|---|
|
Chemical Formula: |
C16H26N2O15P2 |
|---|
| Average Molecular Weight: |
548.3296 |
|---|
| Monoisotopic Molecular
Weight: |
548.080841196 |
|---|
| InChI Key: |
ZOSQFDVXNQFKBY-CZRCVJRHSA-N |
|---|
| InChI: | InChI=1S/C16H26N2O15P2/c1-6-4-18(16(24)17-14(6)23)10-3-8(19)9(31-10)5-29-34(25,26)33-35(27,28)32-15-13(22)12(21)11(20)7(2)30-15/h4,7-13,15,19-22H,3,5H2,1-2H3,(H,25,26)(H,27,28)(H,17,23,24)/t7-,8-,9+,10+,11-,12+,13+,15?/m0/s1 |
|---|
| CAS
number: |
2147-59-3 |
|---|
| IUPAC Name: | {[hydroxy({[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)oxolan-2-yl]methoxy})phosphoryl]oxy}({[(3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy})phosphinic acid |
|---|
|
Traditional IUPAC Name: |
dtdp-L-rhamnose |
|---|
| SMILES: | C[C@@H]1OC(OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H](C[C@@H]2O)N2C=C(C)C(=O)NC2=O)[C@H](O)[C@H](O)[C@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine nucleotide sugars |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine 2'-deoxyribonucleoside diphosphate
- Pyrimidine deoxyribonucleotide
- Organic pyrophosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Pyrimidone
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Hydropyrimidine
- Saccharide
- Heteroaromatic compound
- Vinylogous amide
- Oxolane
- Urea
- Secondary alcohol
- Polyol
- Lactam
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Dong C, Beis K, Giraud MF, Blankenfeldt W, Allard S, Major LL, Kerr ID, Whitfield C, Naismith JH: A structural perspective on the enzymes that convert dTDP-d-glucose into dTDP-l-rhamnose. Biochem Soc Trans. 2003 Jun;31(Pt 3):532-6. Pubmed: 12773151
- Giraud, M. F., Leonard, G. A., Field, R. A., Berlind, C., Naismith, J. H. (2000). "RmlC, the third enzyme of dTDP-L-rhamnose pathway, is a new class of epimerase." Nat Struct Biol 7:398-402. Pubmed: 10802738
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Shibaev, V. N.; Kusov, Yu. Yu.; Eliseeva, G. I.; Petrenko, V. A. Synthesis of thymidine diphosphate rhamnose analogs. Ref. Dokl. Soobshch. - Mendeleevsk. S'ezd Obshch. Prikl. Khim., 11th (1975), 6 111. CODEN: 37MOAO CAN 88:191313 AN 1978:191313 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|