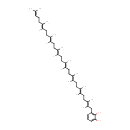
2-Octaprenyl-6-hydroxyphenol (PAMDB000619)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000619 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Octaprenyl-6-hydroxyphenol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2-octaprenyl-6-hydroxyphenol belongs to the class of Tetraterpenes. These are terpene molecules containing 10 consecutively linked isoprene units. (inferred from compound structure)2-octaprenyl-6-hydroxyphenol is invovled in Ubiquinone and other terpenoid-quinone biosynthesis, and Biosynthesis of secondary metabolites. (KEGG) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C48H74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 651.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 650.579052383 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QLVBFESDHCWPKC-WQWYCSGDSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C48H74/c1-38(2)20-12-21-39(3)22-13-23-40(4)24-14-25-41(5)26-15-27-42(6)28-16-29-43(7)30-17-31-44(8)32-18-33-45(9)36-37-48-35-19-34-46(10)47(48)11/h19-20,22,24,26,28,30,32,34-36H,12-18,21,23,25,27,29,31,33,37H2,1-11H3/b39-22+,40-24+,41-26+,42-28+,43-30+,44-32+,45-36+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]benzene-1,2-diol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2-octaprenyl-6-hydroxyphenol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)=CCC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\CC1=C(C)C(C)=CC=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as polyprenylbenzene-1,2-diols. These are compounds containing a polyisoprene chain attached to a catechol group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Polyprenylphenols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Polyprenylbenzene-1,2-diols | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-Octaprenyl-6-hydroxyphenol + S-Adenosylmethionine > 2-Octaprenyl-6-methoxyphenol + S-Adenosylhomocysteine + Hydrogen ion 2-Octaprenylphenol + Oxygen > 2-Octaprenyl-6-hydroxyphenol 2-Octaprenylphenol + Oxygen + NADPH + Hydrogen ion <> 2-Octaprenyl-6-hydroxyphenol + NADP + Water 2-Octaprenyl-6-hydroxyphenol + S-Adenosylmethionine <> 2-Octaprenyl-6-methoxyphenol + S-Adenosylhomocysteine 2-Octaprenylphenol + Hydrogen ion + NADPH + Oxygen + NADPH > NADP + Water + 2-Octaprenyl-6-hydroxyphenol + 2-Octaprenyl-6-hydroxyphenol 2-Octaprenyl-6-hydroxyphenol + S-adenosyl-L-methionine + 2-Octaprenyl-6-hydroxyphenol > Hydrogen ion + S-Adenosylhomocysteine + 2-methoxy-6-(all-trans-octaprenyl)phenol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in 2-polyprenyl-6-methoxy-1,4-benzoquinone methyltransferase activity
- Specific function:
- S-adenosyl-L-methionine + 3- demethylubiquinone-9 = S-adenosyl-L-homocysteine + ubiquinone-9
- Gene Name:
- ubiG
- Locus Tag:
- PA3171
- Molecular weight:
- 25.9 kDa
Reactions
| S-adenosyl-L-methionine + 3-demethylubiquinone-n = S-adenosyl-L-homocysteine + ubiquinone-n. |
| S-adenosyl-L-methionine + 3-(all-trans-polyprenyl)benzene-1,2-diol = S-adenosyl-L-homocysteine + 2-methoxy-6-(all-trans-polyprenyl)phenol. |
- General function:
- Involved in transferase activity, transferring phosphorus-containing groups
- Specific function:
- Required, probably indirectly, for the hydroxylation of 2-octaprenylphenol to 2-octaprenyl-6-hydroxy-phenol, the fourth step in ubiquinone biosynthesis. Specific for aerobically grown log-phase cells
- Gene Name:
- ubiB
- Locus Tag:
- PA5065
- Molecular weight:
- 61.7 kDa