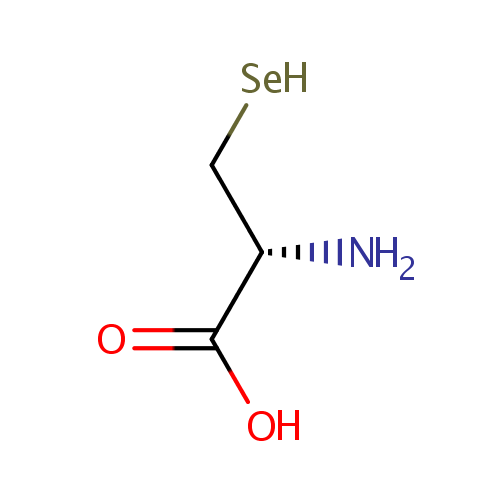
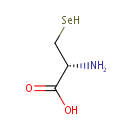| References: |
- Blotcky AJ, Ebrahim A, Rack EP: Determination of selenium metabolites in biological fluids using instrumental and molecular neutron activation analysis. Anal Chem. 1988 Dec 15;60(24):2734-7. Pubmed: 3245598
- Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF: Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood. 1992 Jun 15;79(12):3233-8. Pubmed: 1339300
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Mostert V, Wolff S, Dreher I, Kohrle J, Abel J: Identification of an element within the promoter of human selenoprotein P responsive to transforming growth factor-beta. Eur J Biochem. 2001 Dec;268(23):6176-81. Pubmed: 11733012
- Rooseboom M, Vermeulen NP, Andreadou I, Commandeur JN: Evaluation of the kinetics of beta-elimination reactions of selenocysteine Se-conjugates in human renal cytosol: possible implications for the use as kidney selective prodrugs. J Pharmacol Exp Ther. 2000 Aug;294(2):762-9. Pubmed: 10900258
- Sun QA, Su D, Novoselov SV, Carlson BA, Hatfield DL, Gladyshev VN: Reaction mechanism and regulation of mammalian thioredoxin/glutathione reductase. Biochemistry. 2005 Nov 8;44(44):14528-37. Pubmed: 16262253
- Utomo A, Jiang X, Furuta S, Yun J, Levin DS, Wang YC, Desai KV, Green JE, Chen PL, Lee WH: Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J Biol Chem. 2004 Oct 15;279(42):43522-9. Epub 2004 Aug 4. Pubmed: 15294905
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Zimmermann MB, Kohrle J: The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002 Oct;12(10):867-78. Pubmed: 12487769
- Zinoni, F., Birkmann, A., Stadtman, T. C., Bock, A. (1986). "Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli." Proc Natl Acad Sci U S A 83:4650-4654. Pubmed: 2941757
|
|---|