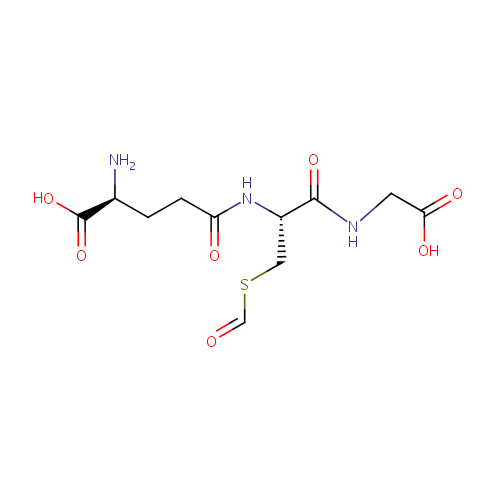
S-Formylglutathione (PAMDB000417)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000417 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | S-Formylglutathione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | S-Formylglutathione is formed from the oxidation of S-hydroxymethylglutathione by the enzyme formaldehyde dehydrogenase (FDH; EC 1.2.1.1) in the presence of NAD (PubMed ID 2806555) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H17N3O7S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 335.334 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 335.078720603 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | FHXAGOICBFGEBF-BQBZGAKWSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H17N3O7S/c12-6(11(20)21)1-2-8(16)14-7(4-22-5-15)10(19)13-3-9(17)18/h5-7H,1-4,12H2,(H,13,19)(H,14,16)(H,17,18)(H,20,21)/t6-,7-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 50409-81-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-(formylsulfanyl)ethyl]carbamoyl}butanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | S-formylglutathione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | N[C@@H](CCC(=O)N[C@@H](CSC=O)C(=O)NCC(O)=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as gamma-glutamyl peptides. These are oligo- and polypeptides consisting of any C-terminal alpha peptide having a gamma-glutamyl residue attached at the N alpha-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Gamma-glutamyl peptides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | S-Formylglutathione + Water <> Formic acid + Glutathione + Hydrogen ion S-(Hydroxymethyl)glutathione + NAD <> S-Formylglutathione + Hydrogen ion + NADH S-Formylglutathione + Water <> Formic acid + Glutathione NAD(P)<sup>+</sup> + S-(Hydroxymethyl)glutathione <> NAD(P)H + S-Formylglutathione + Hydrogen ion S-(Hydroxymethyl)glutathione + NAD(P)(+) > S-Formylglutathione + NAD(P)H S-(Hydroxymethyl)glutathione + NAD + NADP <> S-Formylglutathione + NADH + NADPH + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in zinc ion binding
- Specific function:
- Has high formaldehyde dehydrogenase activity in the presence of glutathione and catalyzes the oxidation of normal alcohols in a reaction that is not GSH-dependent. In addition, hemithiolacetals other than those formed from GSH, including omega-thiol fatty acids, also are substrates
- Gene Name:
- frmA
- Locus Tag:
- PA3629
- Molecular weight:
- 39.2 kDa
Reactions
| S-(hydroxymethyl)glutathione + NAD(P)(+) = S-formylglutathione + NAD(P)H. |
| An alcohol + NAD(+) = an aldehyde or ketone + NADH. |
- General function:
- Involved in carboxylesterase activity
- Specific function:
- Serine hydrolase involved in the detoxification of formaldehyde. Hydrolyzes S-formylglutathione to glutathione and formate. Shows also esterase activity against alpha-naphthyl acetate, lactoylglutathione, palmitoyl-CoA and several pNP-esters of short chain fatty acids
- Gene Name:
- yeiG
- Locus Tag:
- PA3628
- Molecular weight:
- 31.2 kDa
Reactions
| S-formylglutathione + H(2)O = glutathione + formate. |