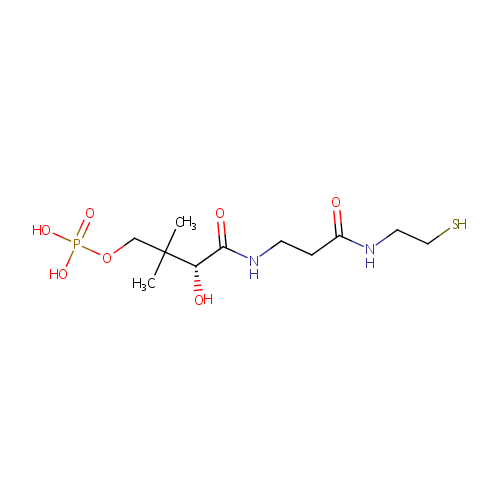
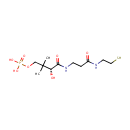Pantetheine 4'-phosphate (PAMDB000379)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000379 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Pantetheine 4'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Pantetheine 4'-phosphate is a metabolite in the pantothenate and coenzyme A biosynthesis pathway. It can be generated from pantatheine (via pantothenate kinase 1) or R-4'-phospho-pantothenoyl-L-cysteine (via phosphopantothenoylcysteine decarboxylase) or dephospho-CoA (via 4'-phosphopantetheine adenylyl-transferase and ectonucleotide pyrophosphatase). The conversion of pantetheine 4'-phosphate (4'-PP) to dephospho-CoA, is catalyzed by 4'-phosphopantetheine adenylyl-transferase. It has been identified as an essential cofactor in in the biosynthesis of fatty acids, polyketides, depsipeptides, peptides, and compounds derived from both carboxylic and amino acid precursors. In particular it is a key prosthetic group of acyl carrier protein (ACP) and peptidyl carrier proteins (PCP) and aryl carrier proteins (ArCP) derived from Coenzyme A. Phosphopantetheine fulfils two demands. Firstly, the intermediates remain covalently linked to the synthases (or synthetases) in an energy-rich thiol ester linkage. Secondly, the flexibility and length of phosphopantetheine chain (approximately 2 nm) allows the covalently tethered intermediates to have access to spatially distinct enzyme active sites. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H23N2O7PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 358.348 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 358.096358302 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | JDMUPRLRUUMCTL-VIFPVBQESA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H23N2O7PS/c1-11(2,7-20-21(17,18)19)9(15)10(16)13-4-3-8(14)12-5-6-22/h9,15,22H,3-7H2,1-2H3,(H,12,14)(H,13,16)(H2,17,18,19)/t9-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2226-71-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(3R)-3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 4'-phosphopantetheine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)(COP(O)(O)=O)[C@@H](O)C(=O)NCCC(=O)NCCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Amino acids, peptides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Beta amino acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Hydrogen ion + Pantetheine 4'-phosphate <> Dephospho-CoA + Pyrophosphate 4-Phosphopantothenoylcysteine + Hydrogen ion <> Carbon dioxide + Pantetheine 4'-phosphate + pantotheine 4'-phosphate Acyl-carrier protein + Water + Acyl-carrier protein <> Pantetheine 4'-phosphate + Apo-[acyl-carrier-protein] Adenosine triphosphate + Pantetheine <> ADP + Pantetheine 4'-phosphate Adenosine triphosphate + Pantetheine 4'-phosphate <> Pyrophosphate + Dephospho-CoA 4-Phosphopantothenoylcysteine <> Pantetheine 4'-phosphate + Carbon dioxide Hydrogen ion + 4-Phosphopantothenoylcysteine > Pantetheine 4'-phosphate + Carbon dioxide Holo-[acyl-carrier-protein] + Water > Pantetheine 4'-phosphate + apo-[acyl-carrier-protein] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Masuda, Toru; Fujii, Shoichiro; Takanohashi, Kunio. Pantetheine 4'-phosphate. Jpn. Tokkyo Koho (1971), 3 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Reversibly transfers an adenylyl group from ATP to 4'- phosphopantetheine, yielding dephospho-CoA (dPCoA) and pyrophosphate
- Gene Name:
- coaD
- Locus Tag:
- PA0363
- Molecular weight:
- 17.8 kDa
Reactions
| ATP + pantetheine 4'-phosphate = diphosphate + 3'-dephospho-CoA. |
- General function:
- Involved in phosphopantothenate--cysteine ligase activity
- Specific function:
- Catalyzes two steps in the biosynthesis of coenzyme A. In the first step cysteine is conjugated to 4'-phosphopantothenate to form 4-phosphopantothenoylcysteine, in the latter compound is decarboxylated to form 4'-phosphopantotheine
- Gene Name:
- coaBC
- Locus Tag:
- PA5320
- Molecular weight:
- 43.1 kDa
Reactions
| N-((R)-4'-phosphopantothenoyl)-L-cysteine = pantotheine 4'-phosphate + CO(2). |
| CTP + (R)-4'-phosphopantothenate + L-cysteine = CMP + diphosphate + N-((R)-4'-phosphopantothenoyl)-L-cysteine. |