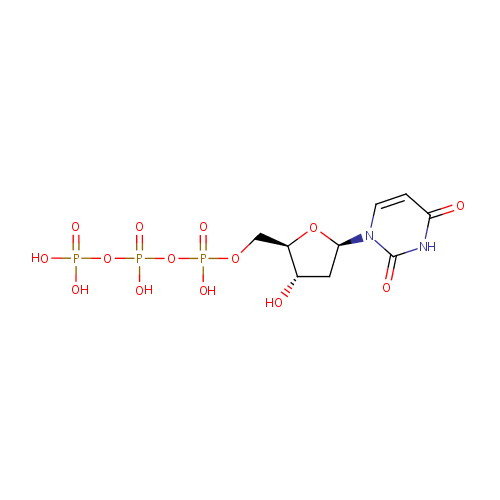
Deoxyuridine triphosphate (PAMDB000290)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000290 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Deoxyuridine triphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Deoxyuridine triphosphate is an intermediate in the metabolism of Pyrimidine. It is a substrate for Inosine triphosphate pyrophosphatase, Uridine-cytidine kinase 1, Nucleoside diphosphate kinase 3, Nucleoside diphosphate kinase B, Nucleoside diphosphate kinase 6, Nucleoside diphosphate kinase homolog 5, Nucleoside diphosphate kinase A and Nucleoside diphosphate kinase 7. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H15N2O14P3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 468.1417 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 467.973612734 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | AHCYMLUZIRLXAA-SHYZEUOFSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H15N2O14P3/c12-5-3-8(11-2-1-7(13)10-9(11)14)23-6(5)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,18,19)(H,20,21)(H,10,13,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 1173-82-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | ({[({[(2R,3S,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | dUTP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H]1C[C@@H](O[C@@H]1COP(O)(=O)OP(O)(=O)OP(O)(O)=O)N1C=CC(=O)NC1=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleoside triphosphates. These are pyrimidine nucleotides with a triphosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine deoxyribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine 2'-deoxyribonucleoside triphosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 Flavodoxin reduced + 2 Hydrogen ion + Uridine triphosphate > Deoxyuridine triphosphate +2 flavodoxin semi oxidized + Water Adenosine triphosphate + dUDP <> ADP + Deoxyuridine triphosphate Deoxyuridine triphosphate + Water <> dUMP + Hydrogen ion + Pyrophosphate dCTP + Hydrogen ion + Water > Deoxyuridine triphosphate + Ammonium Deoxyuridine triphosphate + Thioredoxin disulfide + Water <> Uridine triphosphate + Thioredoxin Deoxyuridine triphosphate + Water <> dUMP + Pyrophosphate Adenosine triphosphate + dUDP <> ADP + Deoxyuridine triphosphate Deoxyuridine triphosphate + Uridine <> dUDP + Uridine 5'-monophosphate Deoxyuridine triphosphate + Cytidine <> dUDP + Cytidine monophosphate Water + dCTP > Ammonia + Deoxyuridine triphosphate dUDP + Adenosine triphosphate + dUDP > Adenosine diphosphate + Deoxyuridine triphosphate + ADP Uridine triphosphate + a reduced flavodoxin + Uridine triphosphate > Water + an oxidized flavodoxin + Deoxyuridine triphosphate Deoxyuridine triphosphate + Water > Phosphate + Hydrogen ion + dUMP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in hydrolase activity
- Specific function:
- This enzyme is involved in nucleotide metabolism:it produces dUMP, the immediate precursor of thymidine nucleotides and it decreases the intracellular concentration of dUTP so that uracil cannot be incorporated into DNA
- Gene Name:
- dut
- Locus Tag:
- PA5321
- Molecular weight:
- 15.9 kDa
Reactions
| dUTP + H(2)O = dUMP + diphosphate. |
- General function:
- Involved in nucleoside diphosphate kinase activity
- Specific function:
- Major role in the synthesis of nucleoside triphosphates other than ATP. The ATP gamma phosphate is transferred to the NDP beta phosphate via a ping-pong mechanism, using a phosphorylated active-site intermediate
- Gene Name:
- ndk
- Locus Tag:
- PA3807
- Molecular weight:
- 15.6 kDa
Reactions
| ATP + nucleoside diphosphate = ADP + nucleoside triphosphate. |
- General function:
- Involved in [formate-C-acetyltransferase]-activating enzyme activity
- Specific function:
- Activation of anaerobic ribonucleoside-triphosphate reductase under anaerobic conditions by generation of an organic free radical, using S-adenosylmethionine and reduced flavodoxin as cosubstrates to produce 5'-deoxy-adenosine
- Gene Name:
- nrdG
- Locus Tag:
- PA1919
- Molecular weight:
- 25.7 kDa
- General function:
- Involved in nucleoside-triphosphate diphosphatase activity
- Specific function:
- Specific function unknown
- Gene Name:
- mazG
- Locus Tag:
- PA0935
- Molecular weight:
- 31.2 kDa
Reactions
| ATP + H(2)O = AMP + diphosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin
- Gene Name:
- nrdD
- Locus Tag:
- PA1920
- Molecular weight:
- 76.1 kDa
Reactions
| 2'-deoxyribonucleoside triphosphate + thioredoxin disulfide + H(2)O = ribonucleoside triphosphate + thioredoxin. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Hydrolyzes O6 atom-containing purine bases deoxyinosine triphosphate (dITP) and xanthosine triphosphate (XTP) as well as 2'-deoxy-N-6-hydroxylaminopurine triposphate (dHAPTP) to nucleotide monophosphate and pyrophosphate. Probably excludes non- standard purines from DNA precursor pool, preventing thus incorporation into DNA and avoiding chromosomal lesions
- Gene Name:
- rdgB
- Locus Tag:
- PA0387
- Molecular weight:
- 21.2 kDa
Reactions
| A nucleoside triphosphate + H(2)O = a nucleotide + diphosphate. |
- General function:
- Involved in ATP binding
- Specific function:
- Catalyzes the reversible transfer of the terminal phosphate group between ATP and AMP. This small ubiquitous enzyme involved in the energy metabolism and nucleotide synthesis, is essential for maintenance and cell growth
- Gene Name:
- adk
- Locus Tag:
- PA3686
- Molecular weight:
- 23.1 kDa
Reactions
| ATP + AMP = 2 ADP. |