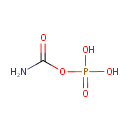
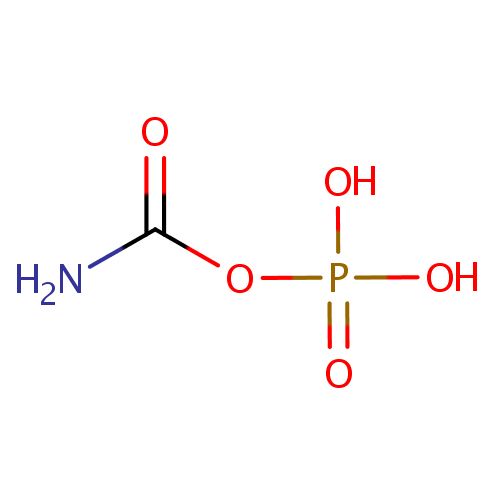
Carbamoylphosphate (PAMDB000253)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000253 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Carbamoylphosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Carbamoyl phosphate is a precursor of both arginine and pyrimidine biosynthesis. It is a labile and potentially toxic intermediate. Carbamoyl phosphate is produced from carbon dioxide, ammonia, and phosphate (from ATP) by the enzyme carbamoyl phosphate synthase. -- Wikipedia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | CH4NO5P | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 141.0199 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 140.982708755 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | FFQKYPRQEYGKAF-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/CH4NO5P/c2-1(3)7-8(4,5)6/h(H2,2,3)(H2,4,5,6) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 590-55-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (carbamoyloxy)phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | carbamoyl-phosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC(=O)OP(O)(O)=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as organic phosphoric acids. These are organic compounds containing phosphoric acid, with the general structure OP(O)(=O)O. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organophosphorus compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Organic phosphoric acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Organic phosphoric acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Aspartic acid + Carbamoylphosphate <> Ureidosuccinic acid + Hydrogen ion + Phosphate 2 Adenosine triphosphate + L-Glutamine + Water + Hydrogen carbonate >2 ADP + Carbamoylphosphate + L-Glutamate +2 Hydrogen ion + Phosphate Adenosine triphosphate + Carbon dioxide + Ammonium <> ADP + Carbamoylphosphate +2 Hydrogen ion Carbamoylphosphate + Ornithine + L-Ornithine <> Citrulline + Hydrogen ion + Phosphate Adenosine triphosphate + Ammonia + Carbon dioxide <> ADP + Carbamoylphosphate 2 Adenosine triphosphate + L-Glutamine + Hydrogen carbonate + Water <>2 ADP + Phosphate + L-Glutamate + Carbamoylphosphate Adenosine triphosphate + Carbamic acid <> ADP + Carbamoylphosphate Carbamoylphosphate + L-Aspartic acid <> Phosphate + Ureidosuccinic acid Carbamoylphosphate + Ornithine <> Phosphate + Citrulline Ammonia + Carbon dioxide + Adenosine triphosphate < Hydrogen ion + Carbamoylphosphate + ADP Ornithine + Carbamoylphosphate <> Hydrogen ion + Citrulline + Phosphate Oxamate + Carbamoylphosphate < Phosphate + Oxalureate Adenosine triphosphate + Hydrogen carbonate + Ammonia > ADP + Phosphate + Carbamoylphosphate + Hydrogen ion 2 Adenosine triphosphate + L-Glutamine + Carbonic acid + Water >2 ADP + Inorganic phosphate + L-Glutamate + Carbamoylphosphate Carbamoylphosphate + Ornithine > Inorganic phosphate + Citrulline Carbamoylphosphate + L-Aspartic acid > Inorganic phosphate + Ureidosuccinic acid 2 Adenosine triphosphate + L-Glutamine + Hydrogen carbonate + Water + Ammonia + Carbamic acid + Carboxyphosphate <>2 ADP + Phosphate + L-Glutamate + Carbamoylphosphate Ornithine + Carbamoylphosphate + Ornithine > Phosphate + Hydrogen ion + Citrulline Hydrogen carbonate + Water + L-Glutamine + 2 Adenosine triphosphate >2 Adenosine diphosphate + Phosphate + L-Glutamic acid +2 Hydrogen ion + Carbamoylphosphate +2 ADP + L-Glutamate Carbamoylphosphate + L-Aspartic acid + L-Aspartic acid > Phosphate + Hydrogen ion + N-carbamoyl-L-aspartate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in ATP binding
- Specific function:
- 2 ATP + L-glutamine + HCO(3)(-) + H(2)O = 2 ADP + phosphate + L-glutamate + carbamoyl phosphate
- Gene Name:
- carB
- Locus Tag:
- PA4756
- Molecular weight:
- 117.3 kDa
Reactions
| 2 ATP + L-glutamine + HCO(3)(-) + H(2)O = 2 ADP + phosphate + L-glutamate + carbamoyl phosphate. |
- General function:
- Involved in carboxyl- or carbamoyltransferase activity
- Specific function:
- Carbamoyl phosphate + L-ornithine = phosphate + L-citrulline
- Gene Name:
- argF
- Locus Tag:
- PA3537
- Molecular weight:
- 33.9 kDa
Reactions
| Carbamoyl phosphate + L-ornithine = phosphate + L-citrulline. |
- General function:
- Involved in glutamine catabolic process
- Specific function:
- 2 ATP + L-glutamine + HCO(3)(-) + H(2)O = 2 ADP + phosphate + L-glutamate + carbamoyl phosphate
- Gene Name:
- carA
- Locus Tag:
- PA4758
- Molecular weight:
- 40.8 kDa
Reactions
| 2 ATP + L-glutamine + HCO(3)(-) + H(2)O = 2 ADP + phosphate + L-glutamate + carbamoyl phosphate. |
- General function:
- Involved in carboxyl- or carbamoyltransferase activity
- Specific function:
- Carbamoyl phosphate + L-aspartate = phosphate + N-carbamoyl-L-aspartate
- Gene Name:
- pyrB
- Locus Tag:
- PA0402
- Molecular weight:
- 36.6 kDa
Reactions
| Carbamoyl phosphate + L-aspartate = phosphate + N-carbamoyl-L-aspartate. |
- General function:
- Involved in cellular amino acid biosynthetic process
- Specific function:
- ATP + NH(3) + CO(2) = ADP + carbamoyl phosphate
- Gene Name:
- arcC
- Locus Tag:
- PA5173
- Molecular weight:
- 33.1 kDa
Reactions
| ATP + NH(3) + CO(2) = ADP + carbamoyl phosphate. |