|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000180 |
|---|
|
Identification |
|---|
| Name: |
Cysteine-S-sulfate |
|---|
| Description: | Cysteine-S-sulfate (SSC) is produced by reaction of inorganic sulfite and cystine by a yet unknown pathway and is a very potent NMDA-receptor agonist. Electrophysiological studies have shown that SSC displays depolarizing properties similar to glutamate. It is an intermediate of cysteine and methionine metabolism. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Alaninethiosulfate
- Alaninethiosulfurate
- Alaninethiosulfuric acid
- Alaninethiosulphate
- Alaninethiosulphurate
- Alaninethiosulphuric acid
- Cysteine-S-sulfate
- Cysteine-S-sulfonate
- Cysteine-S-sulfonic acid
- Cysteine-S-sulfuric acid
- Cysteine-S-sulphate
- Cysteine-S-sulphonate
- Cysteine-S-sulphonic acid
- Cysteine-S-sulphuric acid
- Cysteinyl-S-sulfonate
- Cysteinyl-S-sulfonic acid
- Cysteinyl-S-sulphonate
- Cysteinyl-S-sulphonic acid
- L-Cysteine hydrogen sulfate (ester)
- L-Cysteine hydrogen sulfuric acid (ester)
- L-Cysteine hydrogen sulphate (ester)
- L-Cysteine hydrogen sulphuric acid (ester)
- L-Cysteine S-sulfate
- L-Cysteine S-sulfuric acid
- L-Cysteine S-sulphate
- L-Cysteine S-sulphuric acid
- L-Cysteine-S-sulfonate
- L-Cysteine-S-sulfonic acid
- L-Cysteine-S-sulphonate
- L-Cysteine-S-sulphonic acid
- L-Cysteinesulfonate
- L-Cysteinesulfonic acid
- L-Cysteinesulphonate
- L-Cysteinesulphonic acid
- S-Cysteinesulfonate
- S-Cysteinesulfonic acid
- S-Cysteinesulphonate
- S-Cysteinesulphonic acid
- S-Sulfo-L-cysteine
- S-Sulfocysteine
- S-Sulpho-L-cysteine
- S-Sulphocysteine
|
|---|
|
Chemical Formula: |
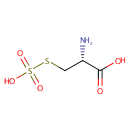
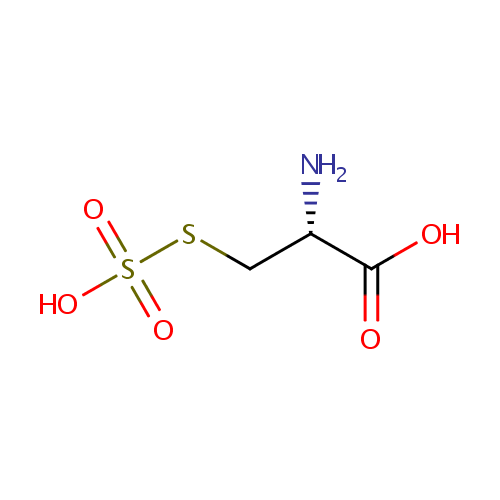
C3H7NO5S2 |
|---|
| Average Molecular Weight: |
201.221 |
|---|
| Monoisotopic Molecular
Weight: |
200.976563719 |
|---|
| InChI Key: |
NOKPBJYHPHHWAN-REOHCLBHSA-N |
|---|
| InChI: | InChI=1S/C3H7NO5S2/c4-2(3(5)6)1-10-11(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9)/t2-/m0/s1 |
|---|
| CAS
number: |
1637-71-4 |
|---|
| IUPAC Name: | (2R)-2-amino-3-(sulfosulfanyl)propanoic acid |
|---|
|
Traditional IUPAC Name: |
S-sulphocysteine |
|---|
| SMILES: | N[C@@H](CSS(O)(=O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
L-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- L-alpha-amino acid
- S-alkyl thiosulfate
- Sulfenyl compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
170-171 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Cysteine and methionine metabolism pae00270
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0930000000-3a10270996044266f5ff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-9100000000-d25cccbc09519d08f684 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-9000000000-285ddd2bd242fb3f4f94 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0090000000-67fc3cbd5242cf8812eb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0080-6920000000-51297f61c1e507e4fc41 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0089-9200000000-a3bb8e3eaa57447953e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-001i-9100000000-7709278c846451c0d981 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-001i-9000000000-b8d9d78e8a4cb67d1d55 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-000i-2920000000-0b87083b8f086069245d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-00di-8910000000-8731b3ae0d4eda8996c3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-00di-9400000000-54f0f85b487cc83c6b80 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0abc-9200000000-7c97c65eb8848afdecb2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0udi-9500000000-a96d24f9946bb7c1a114 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-00di-0900000000-0f68d3c88020aecea493 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-0a6r-9800000000-6d5b706f037dc2097042 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-00dl-9000000000-41e2dd8cc34b88cf9a22 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00di-4910000000-371af0521b2bd90215c3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-008j-7900000000-71cc4905c12f99250ba2 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Abbas AK, Xia W, Tranberg M, Wigstrom H, Weber SG, Sandberg M: S-sulfo-cysteine is an endogenous amino acid in neonatal rat brain but an unlikely mediator of cysteine neurotoxicity. Neurochem Res. 2008 Feb;33(2):301-7. Epub 2007 Sep 1. Pubmed: 17764028
- Arnold GL, Greene CL, Stout JP, Goodman SI: Molybdenum cofactor deficiency. J Pediatr. 1993 Oct;123(4):595-8. Pubmed: 8410516
- Beemer FA, Duran M, Wadman SK, Cats BP: Absence of hepatic molybdenum cofactor. An inborn error of metabolism associated with lens dislocation. Ophthalmic Paediatr Genet. 1985 Apr;5(3):191-5. Pubmed: 3877898
- Duran M, Aarsen G, Fokkens RH, Nibbering NM, Cats BP, de Bree PK, Wadman SK: 2-Mercaptoethanesulfonate-cysteine disulfide excretion following the administration of 2-mercaptoethanesulfonate--a pitfall in the diagnosis of sulfite oxidase deficiency. Clin Chim Acta. 1981 Mar 19;111(1):47-53. Pubmed: 6784974
- Graf WD, Oleinik OE, Jack RM, Weiss AH, Johnson JL: Ahomocysteinemia in molybdenum cofactor deficiency. Neurology. 1998 Sep;51(3):860-2. Pubmed: 9748040
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Rashed MS, Saadallah AA, Rahbeeni Z, Eyaid W, Seidahmed MZ, Al-Shahwan S, Salih MA, Osman ME, Al-Amoudi M, Al-Ahaidib L, Jacob M: Determination of urinary S-sulphocysteine, xanthine and hypoxanthine by liquid chromatography-electrospray tandem mass spectrometry. Biomed Chromatogr. 2005 Apr;19(3):223-30. Pubmed: 15558695
|
|---|
| Synthesis Reference: |
Ubuka T; Kinuta M; Akagi R; Kiguchi S; Azumi M Reaction of S-(2-amino-2-carboxyethylsulfonyl)-L-cysteine with sulfite: synthesis of S-sulfo-L-cysteine and L-alanine 3-sulfinic acid and application to the determination of sulfite. Analytical biochemist |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|