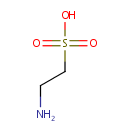
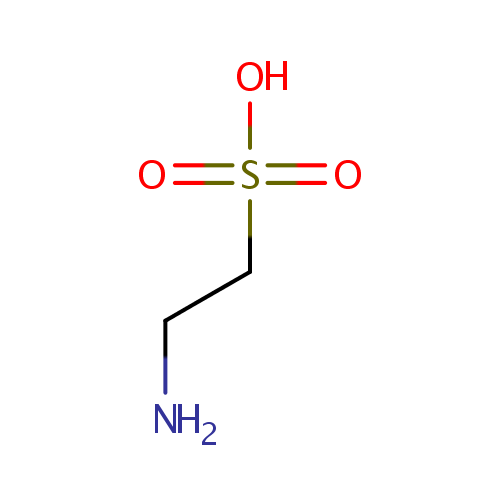
Taurine (PAMDB000106)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000106 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Taurine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Taurine is a sulfur amino acid like methionine, cystine, cysteine and homocysteine. It is a lesser-known amino acid because it is not incorporated into the structural building blocks of protein. Taurine has many diverse biological functions serving as a stabilizer of cell membranes and a facilitator in the transport of ions such as sodium, potassium, calcium and magnesium. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H7NO3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 125.147 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 125.014663785 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | XOAAWQZATWQOTB-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H7NO3S/c3-1-2-7(4,5)6/h1-3H2,(H,4,5,6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 107-35-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-aminoethane-1-sulfonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | taurine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NCCS(O)(=O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as sulfonic acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R is not a hydrogen atom). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Sulfonic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Sulfonic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Sulfonic acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 300 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + Taurine > ADP + Hydrogen ion + Phosphate + Taurine Adenosine triphosphate + Water + Taurine > ADP + Hydrogen ion + Phosphate + Taurine alpha-Ketoglutarate + Oxygen + Taurine <> Aminoacetaldehyde + Carbon dioxide + Hydrogen ion + Sulfite + Succinic acid Cysteic acid <> Taurine + Carbon dioxide (5-L-Glutamyl)-peptide + Taurine <> Peptide + 5-L-Glutamyl-taurine Taurine + alpha-Ketoglutarate + Oxygen <> Sulfite + Aminoacetaldehyde + Succinic acid + Carbon dioxide -->-->Taurine + Oxoglutaric acid + Oxygen > Hydrogen ion + Aminoacetaldehyde + Sulfite + Succinic acid + Carbon dioxide Adenosine triphosphate + Water + Taurine > ADP + Inorganic phosphate + Taurine Adenosine triphosphate + Water + Taurine > ADP + Inorganic phosphate + Taurine Taurine + Oxoglutaric acid + Oxygen > Sulfite + Aminoacetaldehyde + Succinic acid + Carbon dioxide Cysteic acid + Cysteic acid > Taurine + Carbon dioxide Taurine + Oxoglutaric acid + Oxygen > Sulfite + Succinic acid + Aminoacetaldehyde + Carbon dioxide + Sulfite Taurine + Oxoglutaric acid + Oxygen > Sulfite + Succinic acid + Carbon dioxide + Hydrogen ion + Aminoacetaldehyde + Sulfite Taurine + Adenosine triphosphate + Water > Taurine + Adenosine diphosphate + Phosphate + Hydrogen ion + ADP Taurine + Adenosine triphosphate + Water > Taurine + Adenosine diphosphate + Phosphate + Hydrogen ion + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Hu, Libo; Zhu, Hui; Du, Da-Ming; Xu, Jiaxi. Efficient synthesis of taurine and structurally diverse substituted taurines from aziridines. Journal of Organic Chemistry (2007), 72(12), 4543-4546. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in gamma-glutamyltransferase activity
- Specific function:
- (5-L-glutamyl)-peptide + an amino acid = peptide + 5-L-glutamyl amino acid
- Gene Name:
- ggt
- Locus Tag:
- PA1338
- Molecular weight:
- 59.9 kDa
Reactions
| A (5-L-glutamyl)-peptide + an amino acid = a peptide + a 5-L-glutamyl amino acid. |
| Glutathione + H(2)O = L-cysteinylglycine + L-glutamate. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Catalyzes the conversion of taurine and alpha ketoglutarate to sulfite, aminoacetaldehyde and succinate. Required for the utilization of taurine (2-aminoethanesulfonic acid) as an alternative sulfur source. Pentane-sulfonic acid, 3- (N-morpholino)propanesulfonic acid and 1,3-dioxo-2- isoindolineethanesulfonic acid are also substrates for this enzyme
- Gene Name:
- tauD
- Locus Tag:
- PA3935
- Molecular weight:
- 31 kDa
Reactions
| Taurine + 2-oxoglutarate + O(2) = sulfite + aminoacetaldehyde + succinate + CO(2). |
- General function:
- Involved in transporter activity
- Specific function:
- Part of a binding-protein-dependent transport system for taurine. Probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- tauC
- Locus Tag:
- PA3936
- Molecular weight:
- 29.4 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the ABC transporter complex TauABC involved in taurine import. Responsible for energy coupling to the transport system
- Gene Name:
- tauB
- Locus Tag:
- PA3937
- Molecular weight:
- 28.8 kDa
Reactions
| ATP + H(2)O + taurine(Out) = ADP + phosphate + taurine(In). |
- General function:
- Involved in transporter activity
- Specific function:
- Part of a binding-protein-dependent transport system for taurine
- Gene Name:
- tauA
- Locus Tag:
- PA3938
- Molecular weight:
- 35.9 kDa
Transporters
- General function:
- Involved in transporter activity
- Specific function:
- Part of a binding-protein-dependent transport system for taurine. Probably responsible for the translocation of the substrate across the membrane
- Gene Name:
- tauC
- Locus Tag:
- PA3936
- Molecular weight:
- 29.4 kDa
- General function:
- Involved in nucleotide binding
- Specific function:
- Part of the ABC transporter complex TauABC involved in taurine import. Responsible for energy coupling to the transport system
- Gene Name:
- tauB
- Locus Tag:
- PA3937
- Molecular weight:
- 28.8 kDa
Reactions
| ATP + H(2)O + taurine(Out) = ADP + phosphate + taurine(In). |
- General function:
- Involved in transporter activity
- Specific function:
- Part of a binding-protein-dependent transport system for taurine
- Gene Name:
- tauA
- Locus Tag:
- PA3938
- Molecular weight:
- 35.9 kDa