|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000032 |
|---|
|
Identification |
|---|
| Name: |
Glycerophosphocholine |
|---|
| Description: | Glycerophosphorylcholine (GPC) is a choline derivative and one of the two major forms of choline storage (along with phosphocholine) in the cytosol. Glycerophosphorylcholine is also osmolyte. GPC is an intermediate of glycerophospholipid metabolism. It is converted from both 2-acyl-sn-glycero-3-phosphocholine and 1-acyl-sn-glycero-3-phosphocholine by lysophospholipase L2 (EC:3.1.1.5). It is converted to choline and sn-glycerol 3-phosphate by glycerophosphodiester phosphodiesterase (EC:3.1.4.46). (KEGG) |
|---|
|
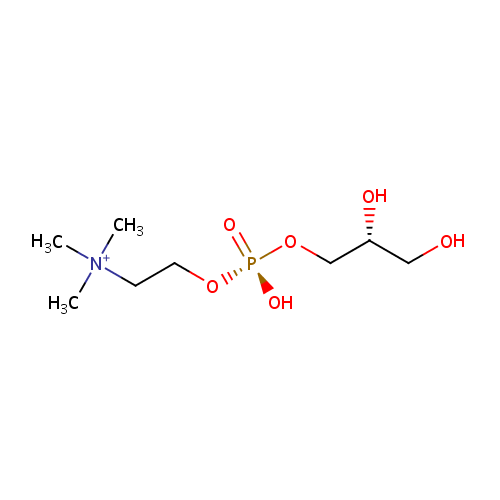
Structure |
|
|---|
| Synonyms: | - 2-[[(2,3-Dihydroxypropoxy)hydroxyphosphinyl]oxy]-N,N,N-trimethyl-Ethanaminium inner salt
- sn-3-GPC
- sn-glycero-3-phosphocholine
- A-Glycerophosphorylcholine
- A-Glycerylphosphorylcholine
- Alpha-Glycerophosphorylcholine
- Alpha-Glycerylphosphorylcholine
- Choline Alfoscerate
- Choline Alfosceric acid
- Choline glycerophosphate
- Choline glycerophosphoric acid
- Glycero-phosphocholine
- Glycerol 3-phosphocholine
- Glycerol phosphorylcholine
- Glycerol-3-phosphatidylcholine
- Glycerophosphatidylcholine
- Glycerophosphocholine
- Glycerophosphorylcholine
- GPC
- GPCho
- Hydrogen glycerophosphate Choline
- Hydrogen glycerophosphoric acid choline
- L-1-Glycero-phosphorylcholine
- L-a-Glycerophosphocholine
- L-a-Glycerophosphorylcholine
- L-a-Glycerylphosphorylcholine
- L-alpha-Glycerophosphocholine
- L-alpha-Glycerophosphorylcholine
- L-alpha-Glycerylphosphorylcholine
- L-Choline hydroxide 2,3-dihydroxypropyl hydrogen phosphate inner salt
- L-Choline hydroxide 2,3-dihydroxypropyl hydrogen phosphoric acid inner salt
- L-α-Glycerophosphocholine
- L-α-Glycerophosphorylcholine
- L-α-Glycerylphosphorylcholine
- Sn-3-GPC
- Sn-Glycero-3-phosphocholine
- α-Glycerophosphorylcholine
- α-Glycerylphosphorylcholine
|
|---|
|
Chemical Formula: |
C8H21NO6P |
|---|
| Average Molecular Weight: |
258.2292 |
|---|
| Monoisotopic Molecular
Weight: |
258.110648921 |
|---|
| InChI Key: |
SUHOQUVVVLNYQR-MRVPVSSYSA-O |
|---|
| InChI: | InChI=1S/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/p+1/t8-/m1/s1 |
|---|
| CAS
number: |
28319-77-9 |
|---|
| IUPAC Name: | [(2R)-2,3-dihydroxypropoxy][2-(trimethylazaniumyl)ethoxy]phosphinic acid |
|---|
|
Traditional IUPAC Name: |
glycerylphosphorylcholine |
|---|
| SMILES: | C[N+](C)(C)CCO[P@](O)(=O)OC[C@H](O)CO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as glycerophosphocholines. These are lipids containing a glycerol moiety carrying a phosphocholine at the 3-position. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Glycerophospholipids |
|---|
| Sub Class | Glycerophosphocholines |
|---|
|
Direct Parent |
Glycerophosphocholines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glycero-3-phosphocholine
- Phosphocholine
- Dialkyl phosphate
- Choline
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Saccharide
- Quaternary ammonium salt
- Secondary alcohol
- 1,2-diol
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Organic cation
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
142.5 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Biosynthesis of unsaturated fatty acids pae01040
- Glycerophospholipid metabolism pae00564
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Evans, Christopher Thomas; McCague, Raymond; Tyrrell, Nicholas David. Preparation of phospholipid-intermediate glycerophosphocholine by a crystallization process. PCT Int. Appl. (1993), 8 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|