Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 961 - 970 of
4373 in total
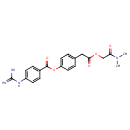
Methanesulfonate (PAMDB001743)
IUPAC:
4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate
CAS: 59721-29-8
Description: Mesylate esters are a group of organic compounds that share a common functional group with the general structure CH3SO2O-R, abbreviated MsO-R, where R is an organic substituent. Mesylate is considered an excellent leaving group in nucleophilic substitution reactions.
Octadecanoate (N-C18:0) (PAMDB001745)
IUPAC:
octadecanoate
CAS: Not Available
Description: Octadecanoate (n-c18:0) belongs to the class of Straight Chain Fatty Acids. These are fatty acids with a straight aliphatic chain. (inferred from compound structure)Stearic acid (STAIR-ik or STEER-ik) is the saturated fatty acid with an 18 carbon chain and has the IUPAC name octadecanoic acid. It is a waxy solid, and its chemical formula is CH3(CH2)16CO2H. Its name comes from the Greek word ???α? st?ar, which means tallow. The salts and esters of stearic acid are called stearates. Stearic acid is one of the most common saturated fatty acids found in nature following palmitic acid. (WikiPedia)
Octadecenoate (N-C18:1) (PAMDB001746)
IUPAC:
(9E)-octadec-9-enoate
CAS: Not Available
Description: Octadecenoate is the salt or easter of octadecenoic acid (oleic acid).
p-Cresol (PAMDB001747)
IUPAC:
4-methylphenol
CAS: 106-44-5
Description: p-Cresol, also known as 4-methylphenol or 4-hydroxytoluene, is a positional isomer of cresol. The other two isomers are m-cresol and o-cresol. (Wikipedia) p-Cresol is one of the metabolites of the amino acid tyrosine, and to a certain extent also of phenylalanine, which are converted to 4-hydroxyphenylacetic acid by intestinal bacteria, before being decarboxylated to p-cresol (putrefaction). The main contributing bacteria are aerobes (mainly enterobacteria), but to a certain extent also anaerobes play a role (mainly Clostridium perfringens). p-Cresol has been reported to affect several biochemical, biological and physiological functions: for example, it alters cell membrane permeability in bacteria. (HMDB, PMID 10570076) In Pseudomonas aeruginosa, p-Cresol can be produced as a by-product of thiazole biosynthesis. (EcoCyc)
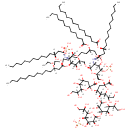
Phospho-heptosyl-heptosyl-kdo2-lipidA (PAMDB001748)
IUPAC:
(2R,4R,5R,6R)-4-{[2-carboxylato-6-(1,2-dihydroxyethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4R,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-5-(phosphonatooxy)oxan-2-yl]oxy}-3,5-dihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-5-{[(3R)-3-(dodecanoyloxy)-1-oxidotetradecylidene]amino}-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-3-(phosphonatooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylate
CAS: Not Available
Description: Phospho-heptosyl-heptosyl-kdo2-lipida belongs to the class of Hexose Oligosaccharides. These are oligosaccharides in which the saccharide units are hexoses. (inferred from compound structure)
Phosphoethanolamine KDO(2)-lipid (A) (PAMDB001750)
IUPAC:
(2-{[1-(6-carboxylato-6-{[2-carboxylato-6-(1,2-dihydroxyethyl)-2-({5-[3-(dodecanoyloxy)tetradecanamido]-6-{[3-hydroxy-5-(3-hydroxytetradecanamido)-4-[(3-hydroxytetradecanoyl)oxy]-6-(phosphonatooxy)oxan-2-yl]methoxy}-3-(phosphonatooxy)-4-{[3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl}methoxy)-5-hydroxyoxan-4-yl]oxy}-3,4-dihydroxyoxan-2-yl)-2-hydroxyethyl phosphonato]oxy}ethyl)azaniumyl
CAS: Not Available
Description: Phosphoethanolamine kdo(2)-lipid (a) belongs to the class of Polysaccharide Phosphates. These are polysaccharides in which a phosphate group is bound to at least one carbohydrate unit. (inferred from compound structure)
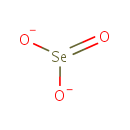
Selenite (PAMDB001752)
IUPAC:
selenite
CAS: 14124-67-5
Description: The selenite anion is a selenium oxoanion with the chemical formula SeO32−. A selenite (compound) is a compound that contains this ion. In slightly acid conditions, the hydrogenselenite ion, HSeO3−, is formed; in more acidic conditions selenous acid, H2SeO3, exists. Most selenite salts can be formed by heating the relevant metal oxide with selenium dioxide, e.g.:Na2O + SeO2 → Na2SeO3. Selenite is an inorganic form of selenium which may also be useful in cancer chemotherapy.(Wikipedia)
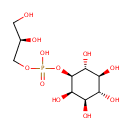
Sn-Glycero-3-phospho-1-inositol (PAMDB001754)
IUPAC:
[(2R)-2,3-dihydroxypropoxy]({[(1S,2R,3R,4S,5S,6R)-2,3,4,5,6-pentahydroxycyclohexyl]oxy})phosphinic acid
CAS: Not Available
Description: Sn-glycero-3-phospho-1-inositol is a member of the chemical class known as Inositol Phosphates. These are compounds containing a phosphate group attached to an inositol (or cyclohexanehexol) moiety.
Sulfoacetate (PAMDB001755)
IUPAC:
2-sulfoacetic acid
CAS: 123-43-3
Description: Sulfoacetate is a member of the chemical class known as Sulfonic Acids. These are compounds containing the sulfonic acid group, which has the general structure RS(=O)2OH (R = H). Sulfoacetate is invovled in Taurine and hypotaurine metabolism. (KEGG)
tetradecanoate (n-C14:0) (PAMDB001757)
IUPAC:
tetradecanoate
CAS: Not Available
Description: Tetradecanoate (n-c14:0) belongs to the class of Straight Chain Fatty Acids. These are fatty acids with a straight aliphatic chain. (inferred from compound structure)