|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001743 |
|---|
|
Identification |
|---|
| Name: |
Methanesulfonate |
|---|
| Description: | Mesylate esters are a group of organic compounds that share a common functional group with the general structure CH3SO2O-R, abbreviated MsO-R, where R is an organic substituent. Mesylate is considered an excellent leaving group in nucleophilic substitution reactions. |
|---|
|
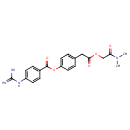
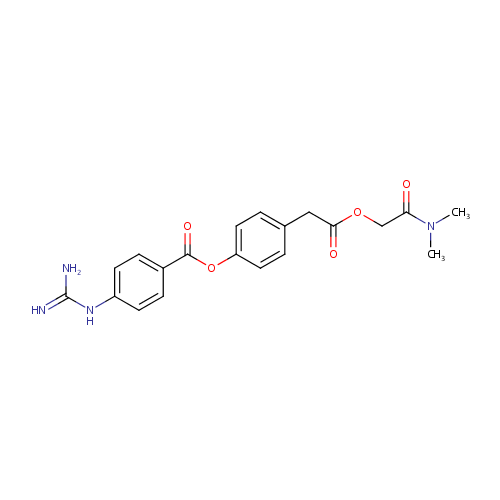
Structure |
|
|---|
| Synonyms: | - Camostat mesilate
- Camostat mesilic acid
- Camostat mesylate
- Camostat mesylic acid
- Camostat monomethanesulfonate
- Camostat monomethanesulfonic acid
- Camostat monomethanesulphonate
- Camostat monomethanesulphonic acid
- Methanesulfonate
- Methanesulfonic acid
- Methanesulphonate
- Methanesulphonic acid
- Methylsulfonate
- Methylsulfonic acid
- Methylsulphonate
- Methylsulphonic acid
- MSA
|
|---|
|
Chemical Formula: |
CH3O3S |
|---|
| Average Molecular Weight: |
95.09 |
|---|
| Monoisotopic Molecular
Weight: |
94.980838711 |
|---|
| InChI Key: |
AFVFQIVMOAPDHO-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/CH4O3S/c1-5(2,3)4/h1H3,(H,2,3,4)/p-1 |
|---|
| CAS
number: |
59721-29-8 |
|---|
| IUPAC Name: | 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate |
|---|
|
Traditional IUPAC Name: |
camostat |
|---|
| SMILES: | CS([O-])(=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as depsides and depsidones. These are polycyclic compounds that is either a polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond (depside), or a compound containing the depsidone structure (depsidone). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Depsides and depsidones |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Depsides and depsidones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Depside backbone
- Guanidinobenzoic acid or derivatives
- Phenylacetate
- Phenol ester
- Benzoate ester
- Aminobenzoic acid or derivatives
- Benzoic acid or derivatives
- Benzoyl
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Tertiary carboxylic acid amide
- Tertiary amine
- Guanidine
- Carboxylic acid ester
- Carboxamide group
- Carboximidamide
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Imine
- Carbonyl group
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|