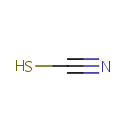Search Results for compounds
Searching compounds for
returned 4373 results.
Tetradecanoyl-phosphate (n-C14:0) (PAMDB001692)
IUPAC:
(tetradecanoyloxy)phosphonate
CAS: Not Available
Description: Tetradecanoyl-phosphate (n-c14:0) belongs to the class of Acyl Phosphates. These are organic compounds containing the functional group -CO-P(O)(O)OH. (inferred from compound structure)
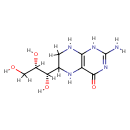
Tetrahydromonapterin (PAMDB001694)
IUPAC:
2-amino-6-[(1S,2S)-1,2,3-trihydroxypropyl]-1,4,5,6,7,8-hexahydropteridin-4-one
CAS: Not Available
Description: Tetrahydromonapterin is a member of the chemical class known as Biopterins and Derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. Tetrahydromonapterin is a major pterin in Pseudomonas aeruginosa and is hypothesized to be the cofactor for phenylalanine hydroxylase (PhhA) in Pseudomonas aeruginosa, but neither its biosynthetic origin nor its cofactor role has been clearly demonstrated. Collectively, these data establish that tetrahydromonapterin formation requires both FolX and FolM, that tetrahydromonapterin is the physiological cofactor for PhhA, and that tetrahydromonapterin can outrank folate as an end product of pterin biosynthesis. (PMID 19897652)
Thiocyanate (PAMDB001695)
IUPAC:
sulfanylformonitrile
CAS: Not Available
Description: Thiocyanate is analogous to the cyanate ion, [OCN]-, wherein oxygen is replaced by sulfur. [SCN]- is one of the pseudohalogens, due to the similarity of its reactions to that of halide ions. Thiocyanate was formerly known as rhodanide (from a Greek word for rose) because of the red color of its complexes with iron. Thiocyanates are typically colorless. Cyanide ions can react with cystine to yield thicocyanate. This reaction occurs to a slight extent even in neutral solution, but is more pronounced in alkaline solutions of cystine. In addition to this non-enzymatic route, cyanide produced in vivo can be converted in part to thiocyanate by sulfur transferase systems. The thiocyanate ion can be oxidized at acid pH by hydrogen peroxide to generate sulfate and cyanide. The reaction is catalyzed by hemoglobin acting as a peroxidase; Thiocyanate is analogous to the cyanate ion, [OCN]-, wherein oxygen is replaced by sulfur. [SCN]- is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide:; Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. As a consequence, thiocyanate can act as a nucleophile at either sulfur or nitrogen
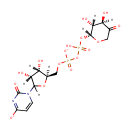
UDP-3-O-(3-Hydroxytetradecanoyl)-D-glucosamine (PAMDB001696)
IUPAC:
1-[(2R,3R,4S,5R)-5-[({[({[(2R,3R,4R,5S,6R)-3-amino-5-hydroxy-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-2-yl]oxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-3,4-dihydroxyoxolan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-olate
CAS: Not Available
Description: UDP-3-o-(3-hydroxytetradecanoyl)-D-glucosamine is a member of the chemical class known as Pyrimidine Nucleotide Sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group.
UDP-4-Keto-pyranose (PAMDB001697)
IUPAC:
1-[(3R,4S,5R)-5-{[({[(2R,3R,4R)-3,4-dihydroxy-5-oxooxan-2-yl]oxy}(hydroxy)phosphoryl phosphonato)oxy]methyl}-3,4-dihydroxyoxolan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-olate
CAS: Not Available
Description: UDP-4-keto-pyranose is a member of the chemical class known as Pyrimidine Ribonucleoside Diphosphates. These are pyrimidine ribobucleotides with diphosphate group linked to the ribose moiety.
Ubiquinone-8 (PAMDB001700)
IUPAC:
2,3-dimethoxy-5-methyl-6-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]cyclohexa-2,5-diene-1,4-dione
CAS: 2394-68-5
Description: Ubiquinone-8 is a member of the chemical class known as Polyprenylbenzoquinones. These are compounds containing a polyisoprene chain attached to a quinone at the second ring position. Ubiquione-8 has isoprene units. Normally in Pseudomonas aeruginosa the active form of Ubiquinone has 8 isoprene units (Ubiquinone-8) and in humans it normally has 10. Ubiquionone is involved in cellular respiration. It is fat-soluble and is therefore mobile in cellular membranes; it plays a unique role in the electron transport chain (ETC). In the inner bacterial membrane, electrons from NADH and succinate pass through the ETC to the oxygen, which is then reduced to water. The transfer of electrons through ETC results in the pumping of H+ across the membrane creating a proton gradient across the membrane, which is used by ATP synthase (located on the membrane) to generate ATP.
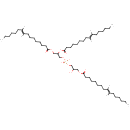
Acyl phosphatidylglycerol (N-C14:1) (PAMDB001706)
IUPAC:
3-({2,3-bis[(7E)-tetradec-7-enoyloxy]propyl phosphonato}oxy)-2-hydroxypropyl (7E)-tetradec-7-enoate
CAS: Not Available
Description: Phosphatidylglycerol is a glycerophospholipid. The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. Glycerophospholipids are important intermediates in cardiolipin synthesis.
Acyl phosphatidylglycerol (N-C16:1) (PAMDB001708)
IUPAC:
1-[(9E)-hexadec-9-enoyloxy]-3-({3-[(9E)-hexadec-9-enoyloxy]-2-hydroxypropyl phosphonato}oxy)propan-2-yl (9E)-hexadec-9-enoate
CAS: Not Available
Description: Phosphatidylglycerol is a glycerophospholipid. The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. Glycerophospholipids are important intermediates in cardiolipin synthesis.
Acyl phosphatidylglycerol (N-C18:1) (PAMDB001710)
IUPAC:
3-({2,3-bis[(11E)-octadec-11-enoyloxy]propyl phosphonato}oxy)-2-hydroxypropyl (11E)-octadec-11-enoate
CAS: Not Available
Description: Phosphatidylglycerol is a glycerophospholipid. The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. Phosphatidylglycerol is important as an intermediate for biosynthesis of other lipids, including cardiolipins.
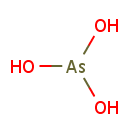
Arsenite (PAMDB001712)
IUPAC:
arsorous acid
CAS: 15502-74-6
Description: Arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3.; In chemistry an arsenite is a chemical compound containing an arsenic oxoanion where arsenic has oxidation state +3. Examples of arsenites include sodium arsenite which contains a polymeric linear anion, [AsO2−]n, silver arsenite, Ag3AsO3, which contains the trigonal, [AsO3]3− anion, sometimes called ortho-arsenite. Arsenite contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure [HPO3]2− and nitrite, NO2− which is planar. Sodium arsenite is used in the water gas shift reaction to remove carbon dioxide.