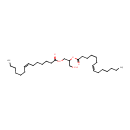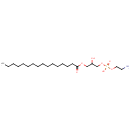
Search Results for compounds
Searching compounds for
returned 4373 results.
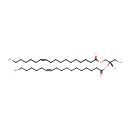
1,2-Diacyl-sn-glycerol (dioctadec-11-enoyl, n-C18:1) (PAMDB001515)
IUPAC:
(2S)-1-hydroxy-3-[(11Z)-octadec-11-enoyloxy]propan-2-yl (11Z)-octadec-11-enoate
CAS: Not Available
Description: 1,2-diacyl-sn-glycerol (dioctadec-11-enoyl, n-c18:1) belongs to the class of Diacylglycerols. These are glycerides consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. (inferred from compound structure)
1,2-Diacyl-sn-glycerol (ditetradec-7-enoyl, n-C14:1) (PAMDB001517)
IUPAC:
1-hydroxy-3-[(7Z)-tetradec-7-enoyloxy]propan-2-yl (7Z)-tetradec-7-enoate
CAS: Not Available
Description: 1,2-diacyl-sn-glycerol (ditetradec-7-enoyl, n-c14:1) belongs to the class of Diacylglycerols. These are glycerides consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. (inferred from compound structure)
1,2-Diacyl-sn-glycerol (ditetradecanoyl, n-C14:0) (PAMDB001518)
IUPAC:
(2S)-1-hydroxy-3-(tetradecanoyloxy)propan-2-yl tetradecanoate
CAS: Not Available
Description: 1,2-diacyl-sn-glycerol (ditetradecanoyl, n-c14:0) belongs to the class of Diacylglycerols. These are glycerides consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. (inferred from compound structure)
1,4-alpha-D-glucan (PAMDB001519)
IUPAC:
Not Available
CAS: 9005-82-7
Description: Amylose is a linear polymer made up of D-glucose units.; Amylose is defined as a linear molecule of (1→4) linked alpha-D-glucopyranosyl units, but it is today well established that some molecules are slightly branched by (1→6)-alpha-linkages. ; The oldest criteria for linearity consisted in the susceptibility of the molecule to complete hydrolysis by beta-amylase. This enzyme splits the (1→4) bonds from the non-reducing end of a chain releasing beta-maltosyl units, but cannot cleave the (1→6) bonds. When degraded by pure beta-amylase, linear macromolecules are completely converted into maltose, whereas branched chains give also one beta-limit dextrin consisting of the remaining inner core polysaccharide structure with its outer chains recessed.; Starches of different botanical origins possess different granular sizes, morphology, polymorphism and enzyme digestibility. These characteristics are related to the chemical structures of the amylopectin and amylose and how they are arranged in the starch granule. (PMID 9730163); Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.; This polysaccharide is one of the two components of starch, making up approximately 20-30% of the structure. The other component is amylopectin, which makes up 70-80% of the structure.
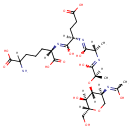
1,6-Anhydrous-N-Acetylmuramyl-tetrapeptide (PAMDB001520)
IUPAC:
(6S)-2-amino-6-{[(2R)-4-carboxy-1-hydroxy-2-{[(2S)-1-hydroxy-2-{[(2R)-1-hydroxy-2-{[(2R,3S,4R,5S)-3-hydroxy-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}propylidene]amino}propylidene]amino}butylidene]amino}-6-[(1-carboxyethyl)-C-hydroxycarbonimidoyl]hexanoic acid
CAS: Not Available
Description: 1,6-Anhydrous-N-Acetylmuramyl-tetrapeptide is an intermediate in peptidoglycan synthesis and turnover. Peptidoglycan can be described as a fisherman's net that encloses bacteria. The mesh of the net is made of two segments of parallel, somewhat inextensible glycan threads, held together by two small elastic peptide crosslinks allowing the net to expand or shrink. The glycan moiety of the peptidoglycan is very uniform among all bacteria, and is made up of alternating β-1,4-linked N-acetylglucosamine and N-acetyl muramate residues, with an average chain lengthof 10 to 65 disaccharide units (depending on the organism). The peptidoglycan synthesis pathway starts in the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-α-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred by an as yet unknown mechanism through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. Peptide crosslinks form between different parts of the peptides depending on the organism. For example, in Mycobacteria and in Pseudomonas aeruginosa most links form between the carboxyl group of the penultimate D-alanine (residue 4) of one peptide to the amino group at the D-center of meso-diaminopimelate (residue 3) of an adjacent peptide of a second glycan chain (as in Pseudomonas aeruginosa). The crosslinking reaction is catalyzed by transpeptidases and involves the cleavage of the D-alanyl-D-alanine bond of the donor peptide, providing the energy to drive the reaction. As a result, the peptides in the peptidoglycan polymers are one or two amino acids shorter than the peptides in the monomers.
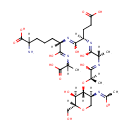
1,6-Anhydrous-N-Acetylmuramyl-tripeptide (PAMDB001521)
IUPAC:
(6S)-2-amino-6-{[(2R)-4-carboxy-1-hydroxy-2-{[(2S)-1-hydroxy-2-{[(2R)-1-hydroxy-2-{[(2R,3S,4R,5S)-3-hydroxy-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}propylidene]amino}propylidene]amino}butylidene]amino}heptanedioic acid
CAS: Not Available
Description: 1,6-anhydrous-n-acetylmuramyl-tripeptide belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure)
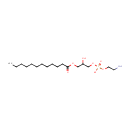
1-Acyl-sn-glycero-3-phosphoethanolamine (N-C12:0) (PAMDB001522)
IUPAC:
(2-aminoethoxy)[3-(dodecanoyloxy)-2-hydroxypropoxy]phosphinic acid
CAS: Not Available
Description: 1-acyl-sn-glycero-3-phosphoethanolamine (n-c12:0) belongs to the class of Lysophosphatidylethanolamines. These are glycerophosphoetahnolamines (molecules containing an ethanolamine moiety attached to the phosphate group linked to a glycerol) with one saturated fatty acid bonded to the glycerol moiety through an ester linkage. (inferred from compound structure)
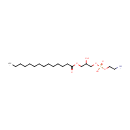
1-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:0) (PAMDB001523)
IUPAC:
(2-aminoethoxy)[2-hydroxy-3-(tetradecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: 1-acyl-sn-glycero-3-phosphoethanolamine (n-c14:0) belongs to the class of Lysophosphatidylethanolamines. These are glycerophosphoetahnolamines (molecules containing an ethanolamine moiety attached to the phosphate group linked to a glycerol) with one saturated fatty acid bonded to the glycerol moiety through an ester linkage. (inferred from compound structure)
1-Acyl-sn-glycero-3-phosphoethanolamine (N-C14:1) (PAMDB001524)
IUPAC:
{2-[(2-hydroxy-3-{[(7E)-1-(??-oxidanyliumylidene)tetradec-7-en-1-yl]oxy}propyl phosphonato)oxy]ethyl}azanylidene
CAS: Not Available
Description: 1-Acyl-sn-glycero-3-phosphoethanolamine (n-C14:1) belongs to the class of Lysophosphatidylethanolamines. These are glycerophosphoetahnolamines (molecules containing an ethanolamine moiety attached to the phosphate group linked to a glycerol) with one saturated fatty acid bonded to the glycerol moiety through an ester linkage. (inferred from compound structure)
1-Acyl-sn-glycero-3-phosphoethanolamine (N-C16:0) (PAMDB001525)
IUPAC:
(2-aminoethoxy)[3-(hexadecanoyloxy)-2-hydroxypropoxy]phosphinic acid
CAS: Not Available
Description: 1-acyl-sn-glycero-3-phosphoethanolamine (n-c16:0) belongs to the class of Lysophosphatidylethanolamines. These are glycerophosphoetahnolamines (molecules containing an ethanolamine moiety attached to the phosphate group linked to a glycerol) with one saturated fatty acid bonded to the glycerol moiety through an ester linkage. (inferred from compound structure)