|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001519 |
|---|
|
Identification |
|---|
| Name: |
1,4-alpha-D-glucan |
|---|
| Description: | Amylose is a linear polymer made up of D-glucose units.; Amylose is defined as a linear molecule of (1→4) linked alpha-D-glucopyranosyl units, but it is today well established that some molecules are slightly branched by (1→6)-alpha-linkages. ; The oldest criteria for linearity consisted in the susceptibility of the molecule to complete hydrolysis by beta-amylase. This enzyme splits the (1→4) bonds from the non-reducing end of a chain releasing beta-maltosyl units, but cannot cleave the (1→6) bonds. When degraded by pure beta-amylase, linear macromolecules are completely converted into maltose, whereas branched chains give also one beta-limit dextrin consisting of the remaining inner core polysaccharide structure with its outer chains recessed.; Starches of different botanical origins possess different granular sizes, morphology, polymorphism and enzyme digestibility. These characteristics are related to the chemical structures of the amylopectin and amylose and how they are arranged in the starch granule. (PMID 9730163); Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.; This polysaccharide is one of the two components of starch, making up approximately 20-30% of the structure. The other component is amylopectin, which makes up 70-80% of the structure. |
|---|
|
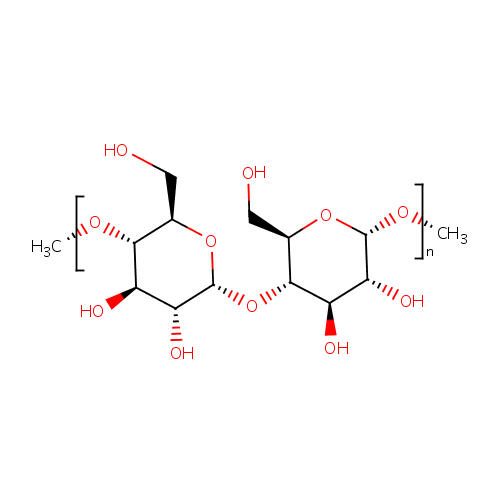
Structure |
|
|---|
| Synonyms: | - (1,4-a-D-Glucosyl)N
- (1,4-a-D-Glucosyl)n+1
- (1,4-a-D-Glucosyl)N-1
- (1,4-a-delta-Glucosyl)N
- (1,4-a-delta-Glucosyl)n+1
- (1,4-a-delta-Glucosyl)N-1
- (1,4-a-δ-Glucosyl)N
- (1,4-a-δ-Glucosyl)n+1
- (1,4-a-δ-Glucosyl)N-1
- (1,4-alpha-D-Glucosyl)N
- (1,4-alpha-D-Glucosyl)N+1
- (1,4-alpha-D-Glucosyl)N-1
- (1,4-alpha-delta-Glucosyl)N
- (1,4-alpha-delta-Glucosyl)N+1
- (1,4-alpha-delta-Glucosyl)N-1
- (1,4-α-D-Glucosyl)N
- (1,4-α-D-Glucosyl)n+1
- (1,4-α-D-Glucosyl)N-1
- (1,4-α-δ-Glucosyl)N
- (1,4-α-δ-Glucosyl)n+1
- (1,4-α-δ-Glucosyl)N-1
- 1,4-a-D-Glucan
- 1,4-a-delta-Glucan
- 1,4-a-δ-Glucan
- 1,4-alpha-D-Glucan
- 1,4-alpha-delta-Glucan
- 1,4-α-D-Glucan
- 1,4-α-δ-Glucan
- 4-{(1,4)-a-D-glucosyl}(N-1)-D-glucose
- 4-{(1,4)-a-delta-glucosyl}(N-1)-delta-glucose
- 4-{(1,4)-a-δ-glucosyl}(N-1)-δ-glucose
- 4-{(1,4)-alpha-D-Glucosyl}(N-1)-D-glucose
- 4-{(1,4)-alpha-delta-Glucosyl}(N-1)-delta-glucose
- 4-{(1,4)-α-D-glucosyl}(N-1)-D-glucose
- 4-{(1,4)-α-δ-glucosyl}(N-1)-δ-glucose
- Amylose
- Amylose chain
- Maltodextrin (NF)
|
|---|
|
Chemical Formula: |
(C12H20O11)nC2H6 |
|---|
| Average Molecular Weight: |
Not Available |
|---|
| Monoisotopic Molecular
Weight: |
Not Available |
|---|
| InChI Key: |
PTHCMJGKKRQCBF-OLYDTGNASA-N |
|---|
| InChI: | InChI=1S/C14H26O11/c1-21-11-5(3-15)24-14(10(20)7(11)17)25-12-6(4-16)23-13(22-2)9(19)8(12)18/h5-20H,3-4H2,1-2H3/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14-/m1/s1 |
|---|
| CAS
number: |
9005-82-7 |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | Not Available |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Glycosyl compounds |
|---|
|
Direct Parent |
O-glycosyl compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- O-glycosyl compound
- Disaccharide
- Oxane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | Not Available |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | Adenosine triphosphate + Water + 1,4-alpha-D-glucan > 1,4-alpha-D-glucan + ADP + Hydrogen ion + PhosphateAdenosine triphosphate + Water + 1,4-alpha-D-glucan > 1,4-alpha-D-glucan + ADP + Hydrogen ion + Phosphate1,4-alpha-D-glucan > Maltohexaose1,4-alpha-D-glucan <> StarchStarch + Phosphate <> 1,4-alpha-D-glucan + Glucose 1-phosphateADP-Glucose + 1,4-alpha-D-glucan <> ADP + 1,4-alpha-D-glucanADP-Glucose + 1,4-alpha-D-glucan <> ADP + 1,4-alpha-D-glucan1,4-alpha-D-glucan + D-Glucose <> D-Maltose |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Buleon, A., Colonna, P., Planchot, V., Ball, S. (1998). "Starch granules: structure and biosynthesis." Int J Biol Macromol 23:85-112. Pubmed: 9730163
- Klein B, Foreman JA: Amylolysis of a chromogenic substrate, Cibachron Blue F3GA-amylose: kinetics and mechanism. Clin Chem. 1980 Feb;26(2):250-3. Pubmed: 6153298
- Langkilde AM, Ekwall H, Bjorck I, Asp NG, Andersson H: Retrograded high-amylose corn starch reduces cholic acid excretion from the small bowel in ileostomy subjects. Eur J Clin Nutr. 1998 Nov;52(11):790-5. Pubmed: 9846590
- Topping DL, Gooden JM, Brown IL, Biebrick DA, McGrath L, Trimble RP, Choct M, Illman RJ: A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. J Nutr. 1997 Apr;127(4):615-22. Pubmed: 9109613
- Traub WH: Studies on neutralization of human serum bactericidal activity by sodium amylosulfate. J Clin Microbiol. 1977 Aug;6(2):128-31. Pubmed: 330560
|
|---|
| Synthesis Reference: |
Breitinger, Hans-Georg. Synthesis and characterization of 2,3-di-O-alkylated amyloses: Hydrophobic substitution destabilizes helical conformation. Biopolymers (2003), 69(3), 301-310. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|