Search Results for compounds
Searching compounds for
returned 4373 results.
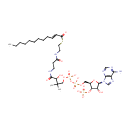
(2E)-Dodecenoyl-CoA (PAMDB000522)
IUPAC:
(3R)-3-{[2-({2-[(2E)-dodec-2-enoylsulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-3-hydroxy-2,2-dimethylpropyl ({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonatooxy)oxolan-2-yl]methyl phosphonato}oxy)phosphonate
CAS: 1066-12-2
Description: (2E)-Dodecenoyl-CoA is an intermediate in fatty acid metabolism, the substrate of the enzyme acyl-CoA oxidase [EC-1.3.3.6], and enzymes acyl-CoA dehydrogenase, long-chain-acyl-CoA dehydrogenase [EC 1.3.99.3-1.3.99.13]. It is also an intermediate in fatty acid elongation, being the substrate of the enzyme enoyl-CoA hydratase and [EC 4.2.1.17]. (KEGG)
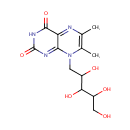
6,7-Dimethyl-8-(1-D-ribityl)lumazine (PAMDB000524)
IUPAC:
6,7-dimethyl-8-(2,3,4,5-tetrahydroxypentyl)-2,3,4,8-tetrahydropteridine-2,4-dione
CAS: 5118-16-1
Description: 6,7-Dimethyl-8-(1-D-ribityl)lumazine is an intermediate in riboflavin metabolism. 6,7-Dimethyl-8-(1-D-ribityl)lumazine is the second to last step in the synthesis of ribitol and is converted from 4-(1-D-ribitylamino)-5-amino-2,6-dihydroxypyrimidine via the enzyme riboflavin synthase beta chain. It is then
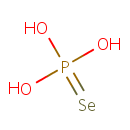
Phosphoroselenoic acid (PAMDB000525)
IUPAC:
hydroxy(selanylidene)phosphonous acid
CAS: 25758-66-1
Description: Phosphoroselenoic acid, H3SePO3, is the activated selenium donor compound required for the biosynthesis of selenocysteyl-tRNA, the precursor of specific selenocysteine residues in bacterial and mammalian selenoproteins. Selenocysteine is often called the 21st amino acid, because Sec has a specific tRNA and codon UGA, and shares a major stop codon UGA. A number of enzymes have selenocysteine, residues and in some cases at their active sites. Proteins containing the 21st amino acid, selenocysteine (Sec), have been described in all three domains of life.
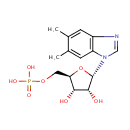
N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole (PAMDB000527)
IUPAC:
{[(2R,3S,4R,5S)-5-(5,6-dimethyl-1H-1,3-benzodiazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid
CAS: Not Available
Description: N1-(5-Phospho-alpha-D-ribosyl)-5,6-dimethylbenzimidazole (or alpha-ribazole-5'-Phosphate) is an intermediate in Riboflavin metabolism. In particular, alpha-Ribazole 5'-phosphate is converted from Dimethylbenzimidazole via the enzyme nicotinate-nucleotide-dimethylbenzimidazole
(S)-3-Hydroxyhexadecanoyl-CoA (PAMDB000529)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxyhexadecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 35106-50-4
Description: (S)-3-Hydroxyhexadecanoyl-CoA is a beta-oxidation intermediate derivative of palmitoyl-CoA and the substrate of the enzyme long chain-fatty acyl-CoA Ligase. This enzyme catalyzes the esterification, concomitant with transport, of exogenous long-chain fatty acids into metabolically active CoA thioesters for subsequent degradation or incorporation into phospholipids. This compound is also a substrate for 3-ketoacyl-CoA thiolase. This enzyme catalyzes the final step of fatty acid oxidation in which acetyl-CoA is released and the CoA ester of a fatty acid two carbons shorter is formed.
(S)-3-Hydroxytetradecanoyl-CoA (PAMDB000530)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxytetradecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: Not Available
Description: (S)-3-Hydroxytetradecanoyl-CoA is an intermediate in Fatty acid elongation. (S)-3-Hydroxytetradecanoyl-CoA is the 7th to last step in the synthesis of Hexadecanoic acid and is converted from 3-Oxotetradecanoyl-CoA via the enzyme long-chain 3-hydroxyacyl-CoA dehydrogenase (EC 1.1.1.211). It is then converted to trans-Tetradec-2-enoyl-CoA via the enzyme enoyl-CoA hydratase (EC 4.2.1.17).
3-Oxotetradecanoyl-CoA (PAMDB000531)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-2,2-dimethyl-3-{[2-({2-[(3-oxotetradecanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 122364-86-7
Description: 3-Oxotetradecanoyl-CoA is a product of the peroxisomal beta oxidation of hexadenoic acid by the enzyme acyl-CoA oxidase which results in long-chain 3-oxoacyl-CoA-esters.
(S)-3-Hydroxydodecanoyl-CoA (PAMDB000532)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxydodecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 72059-49-5
Description: (S)-3-Hydroxydodecanoyl-CoA is a metabolite involved in the fatty acid elongation. The enzyme long-chain-3-hydroxyacyl-CoA dehydrogenase catalyzes the conversion of 3-Oxododecanoyl-CoA to (S)-3-Hydroxydodecanoyl-CoA.
3-Oxododecanoyl-CoA (PAMDB000533)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy(3-hydroxy-2,2-dimethyl-3-{[2-({2-[(3-oxododecanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy)phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 78303-19-2
Description: 3-Oxododecanoyl-CoA is a metabolite involved in the fatty acid elongation. The enzyme acetyl-CoA C-acyltransferase catalyzes the formation of this metabolite from Acetyl-CoA.
(S)-Hydroxydecanoyl-CoA (PAMDB000534)
IUPAC:
{[(2R,3R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxydecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 6245-70-1
Description: (S)-Hydroxydecanoyl-CoA has a role in the synthesis and oxidation of fatty acids. It is involved in fatty acid elongation. In this pathway 3-Oxodecanoyl-CoA is acted upon by two enzymes, 3-hydroxyacyl-CoA dehydrogenase and long-chain-3-hydroxyacyl-CoA dehydrogenase to produce (S)-Hydroxydecanoyl-CoA. Since coenzyme A is chemically a thiol, it can react with carboxylic acids to form thioesters, thus functioning as an acyl group carrier. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA. When it is not attached to an acyl group it is usually referred to as CoASH or HSCoA.