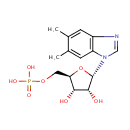
N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole (PAMDB000527)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000527 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | N1-(5-Phospho-alpha-D-ribosyl)-5,6-dimethylbenzimidazole (or alpha-ribazole-5'-Phosphate) is an intermediate in Riboflavin metabolism. In particular, alpha-Ribazole 5'-phosphate is converted from Dimethylbenzimidazole via the enzyme nicotinate-nucleotide-dimethylbenzimidazole | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C14H19N2O7P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 358.2836 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 358.092987484 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ZMRGXEJKZPRBPJ-SYQHCUMBSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C14H19N2O7P/c1-7-3-9-10(4-8(7)2)16(6-15-9)14-13(18)12(17)11(23-14)5-22-24(19,20)21/h3-4,6,11-14,17-18H,5H2,1-2H3,(H2,19,20,21)/t11-,12-,13-,14+/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,4R,5S)-5-(5,6-dimethyl-1H-1,3-benzodiazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | α-ribazole-5'-P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=CC2=C(C=C1C)N(C=N2)[C@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as benzimidazole ribonucleosides and ribonucleotides. These are nucleosides with a structure that consists of an imidazole moiety of benzimidazole is N-linked to a ribose (or deoxyribose). Nucleotides have a phosphate group linked to the C5 carbon of the ribose (or deoxyribose) moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzimidazole ribonucleosides and ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Benzimidazole ribonucleosides and ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Dimethylbenzimidazole + Nicotinamide ribotide <> N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole + Hydrogen ion + Nicotinic acid N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole + Water <> N1-(alpha-D-ribosyl)-5,6-dimethyl-benzimidazole + Phosphate Adenosylcobinamide-GDP + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole > adenosylcobalamin 5'-phosphate + Guanosine monophosphate + Hydrogen ion Nicotinamide ribotide + Dimethylbenzimidazole > Nicotinic acid + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole Adenosylcobinamide-GDP + N1-(alpha-D-ribosyl)-5,6-dimethyl-benzimidazole + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole <> Guanosine monophosphate + Adenosylcobalamin + Adenosylcobalamin 5'-phosphate Dimethylbenzimidazole + nicotinate beta-D-ribonucleotide + Nicotinamide ribotide > Hydrogen ion + Nicotinic acid + N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole N1-(5-Phospho-a-D-ribosyl)-5,6-dimethylbenzimidazole + Adenosylcobinamide-GDP + Adenosylcobinamide-GDP > Hydrogen ion + GMP + Adenosylcobalamin 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||