Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3731 - 3740 of
4373 in total
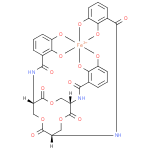
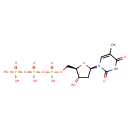
ferric enterobactin complex (PAMDB110841)
IUPAC:
{N,N',N''- [(3S,7S,11S)-
[(3S,7S,11S)- 2,6,10-
2,6,10- trioxo-
trioxo- 1,5,9-
1,5,9- trioxacyclododecane-
trioxacyclododecane- 3,7,11-
3,7,11- triyl]tris[2,3-
triyl]tris[2,3- di(hydroxy-
di(hydroxy- κO)benzamidato]}ferrate(3−)
κO)benzamidato]}ferrate(3−)
CAS: Not Available
Description: Not Available
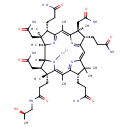
cobinamide (PAMDB110842)
IUPAC:
[(1R,2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-hydroxypropyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltbis(ylium)
CAS: 13497-85-3
Description: Cobinamide is an intermediate in porphyrin and chlorophyll metabolism. It is converted to adenosyl cobinamide via the enzyme cob(I)alamin adenosyltransferase [EC:2.5.1.17]. Adenosyl cobinamide is the third to last step in the synthesis of vitamin B12 coenzyme.
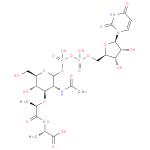
UDP-N-acetyl-α-D-muramoyl-L-alanine (PAMDB110844)
IUPAC:
uridine 5'- (3-
(3- {2-
{2- acetylamino-
acetylamino- 3-
3- O-
O- [2-
[2- (L-
(L- alaninocarboxy)ethyl]-
alaninocarboxy)ethyl]- 2-
2- deoxy-
deoxy- D-
D- glucopyranosyl}dihydrogen diphosphate)
glucopyranosyl}dihydrogen diphosphate)
CAS: Not Available
Description: Not Available
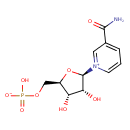
β-nicotinamide D-ribonucleotide (PAMDB110845)
IUPAC:
3-carbamoyl-1-(5-O-phosphonato-β-D-ribofuranosyl)pyridinium
CAS: 1094-61-7
Description: Nicotinamide ribotide (NMN) is an important intermediate metabolite in the nicotinate and nicotinamide metabolism pathway. Mammals predominantly use nicotinamide rather than nicotinic acid as a precursor for NAD biosynthesis. Instead of the deamidation to nicotinic acid, nicotinamide is directly converted to NMN by nicotinamide phosphoribosyltransferase (NAMPT, EC 2.4.2.12). The enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT, EC 2.7.7.1), which is a member of the nucleotidyltransferase alpha/beta-phosphodiesterase superfamily, catalyzes the reaction NMN + ATP <=> Nicotinamide adenine dinucleotide (NAD) + PPi, representing the final step in the biosynthesis of NAD. NAD is a molecule that plays a fundamental role as a cofactor in cellular redox reactions. Thus NMN is an important metabolite for the maintenance of normal NAD biosynthesis. Circulating NMN levels may play an important role in regulating cell function in physiological and pathophysiological conditions. (PMID: 15078171 , 17983582 ).
dTTP (PAMDB110846)
IUPAC:
thymidine 5'-triphosphate(4−)
CAS: 365-08-2
Description: Thymidine triphosphate or TTP is one of the four nucleoside triphosphates that make up DNA. It can be used by DNA ligase to create overlapping "sticky ends" so that protruding ends of opened microbial plasmids maybe closed up.
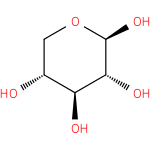
β-D-xylopyranose (PAMDB110847)
IUPAC:
β-D-xylopyranose
CAS: Not Available
Description: Not Available
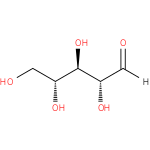
aldehydo-D-ribose (PAMDB110848)
IUPAC:
aldehydo-D-ribo-pentose
CAS: Not Available
Description: Not Available
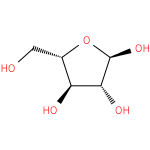
α-L-arabinofuranose (PAMDB110849)
IUPAC:
α-L-arabinofuranose
CAS: Not Available
Description: Not Available
D-erythrose 4-phosphate (PAMDB110850)
IUPAC:
(2R,3R)-2,3-dihydroxy-4-oxobutyl phosphate
CAS: 585-18-2
Description: D-Erythrose 4-phosphate is a phosphorylated derivative of erythrose that serves as an important intermediate in the pentose phosphate pathway. It is also used in phenylalanine, tyrosine and tryptophan biosynthesis, and it plays a role in vitamin B6 metabolism (KEGG).
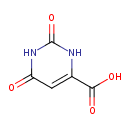
orotate (PAMDB110851)
IUPAC:
2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylate
CAS: 65-86-1
Description: Orotic acid is a minor dietary constituent. Indeed, until it was realized that it could be synthesized by humans, orotic acid was known as vitamin B-13. The richest dietary sources are cow's milk and other dairy products as well as root vegetables such as carrots and beets. Dietary intake probably contributes to a basal rate of orotic acid excretion in urine because fasting decreases excretion by ~50%. However, it is now apparent that most urinary orotic acid is synthesized in the body, where it arises as an intermediate in the pathway for the synthesis of pyrimidine nucleotides. Orotic acid is converted to UMP by UMP synthase, a multifunctional protein with both orotate phosphoribosyltransferase and orotidylate decarboxylase activity. The most frequently observed inborn error of pyrimidine nucleotide synthesis is a mutation of the multifunctional protein UMP synthase. This disorder prevents the conversion of orotic acid to UMP and thus to other pyrimidines. As a result, plasma orotic acid accumulates to high concentrations, and increased quantities appear in the urine. Indeed, urinary orotic acid is so markedly increased in individuals harboring a mutation in UMP synthase that orotic acid crystals can form in the urine. The urinary concentration of orotic acid in homozygotes can be of the order of millimoles per millimole creatinine. By comparison, the urinary level in unaffected individuals is ~ 1 umol/mmol creatinine. (PMID: 17513443 ).