Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3231 - 3240 of
4373 in total
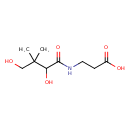
(R)-pantothenate (PAMDB110214)
IUPAC:
3-[(2R)-2,4-dihydroxy-3,3-dimethylbutanamido]propanoate
CAS: 79-83-4
Description: A pantothenate that is the conjugate base of (R)-pantothenic acid, obtained by deprotonation of the carboxy group.
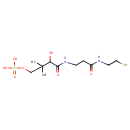
4'-phosphopantetheine (PAMDB110215)
IUPAC:
N3- [(2R)-
[(2R)- 2-
2- hydroxy-
hydroxy- 3,3-
3,3- dimethyl-
dimethyl- 4-
4- (phosphonatooxy)butanoyl]-
(phosphonatooxy)butanoyl]- N-
N- (2-
(2- sulfanylethyl)-
sulfanylethyl)- β-
β- alaninamide
alaninamide
CAS: 2226-71-3
Description: Pantetheine 4'-phosphate(2−) with D (R) configuration at the 2' position. The dianion formed from D-pantetheine 4'-phosphate by deprotonation of the phosphate group; major microspecies at pH 7.3.
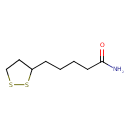
lipoamide (PAMDB110217)
IUPAC:
5-(1,2-dithiolan-3-yl)pentanamide
CAS: 940-69-2
Description: A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of lipoic acid with ammonia.
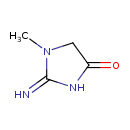
creatinine (PAMDB110219)
IUPAC:
2-imino-1-methylimidazolidin-4-one
CAS: 60-27-5
Description: A lactam obtained by formal cyclocondensation of creatine. It is a metabolite of creatine.
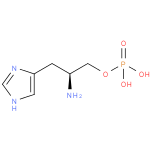
L-histidinol-phosphate (PAMDB110220)
IUPAC:
(2S)-2-amino-3-(1H-imidazol-4-yl)propyl dihydrogen phosphate
CAS: Not Available
Description: The O-phospho derivative of L-histidinol.
Co2+ (PAMDB110228)
IUPAC:
cobalt(II) cation
CAS: 7440-48-4
Description: Cobalt has a molecular weight of 58.9 and an atomic number of 27. In the Periodic Table, close to other transition metals, it is situated in group 8, together with rhodium and iridium and it can occur in four oxidation states (0, +2, +3 and +4). The +2 and the ground state are the most common. Cobalt occurs in the minerals cobaltite (Co, Fe) AsS, smaltite (CoAs2), and erythrite Co3(AsO4)2.8H2O, and is often associated with nickel, silver, lead, copper, and iron ores, from which it is most frequently obtained as a by-product. Depending on the considered species, cobalt has multiple industrial applications including the production of alloys and hard metal, diamond polishing, drying agents, pigments and catalysts. Hard metal or cemented carbide is a powder metallurgical product consisting of hard, wear-resistant carbide particles bound together (cemented) with a ductile metal binder (i.e. metallic Co) by liquid phase sintering. Tungsten carbide (WC) is produced by mixing tungsten powder with pure carbon powder at high temperature; hereafter WC is mixed with Co powder to which paraffin is added as a binder. Depending on specific requirements related to their use, hard metals might additionally contain small quantities of chromium, niobium, molybdenum, titanium, tantalum or vanadium carbides. Inhalation and skin contact are the main occupational exposure routes. Occupational exposure to cobalt may result in adverse health effects in different organs or tissues, including the respiratory tract, the skin, the hemapoietic tissues, the myocardium or the thyroid gland. In addition, teratogenic and carcinogenic effects have been observed in experimental systems and/or in humans. For the general population, the diet constitutes the main route of exposure to cobalt, since it is an essential component of Vitamin B12 (hydroxycolalamin). Cobalt functions as a co-factor in enzyme catalysed reactions and is involved in the production of erythropoietin, a hormone that stimulates the formation of erythrocytes. This last property of cobalt was applied in the past as a therapy for anaemia. The carcinogenic potential of cobalt and its compounds was evaluated in 1991 by the International Agency for Research on Cancer (IARC), which concluded that there was inadequate evidence for carcinogenicity in humans (lung cancer) but sufficient evidence in experimental animal studies. In most experimental studies considered, the routes of exposure were, however, of questionable relevance for cancer risk assessment in humans for example, local sarcomas after intra-muscular injection. The general conclusion was that cobalt and its compounds are possibly carcinogenic to humans (group 2B). Since this evaluation, additional data have been accumulated which generally indicate that, depending on the considered cobalt species, different outcomes regarding toxicity, mutagenicity and carcinogenicity can be observed. Physiologically, it exists as an ion in the body. Co(II) ions are genotoxic in vitro and in vivo, and carcinogenic in rodents. Co metal is genotoxic in vitro. Hard metal dust, of which occupational exposure is linked to an increased lung cancer risk, is proven to be genotoxic in vitro and in vivo. Possibly, production of active oxygen species and/or DNA repair inhibition are mechanisms involved. Given the recently provided proof for in vitro and in vivo genotoxic potential of hard metal dust, the mechanistic evidence of elevated production of active oxygen species and the epidemiological data on increased cancer risk, it may be advisable to consider the possibility of a new evaluation by IARC.(PMID: 14643417 ).
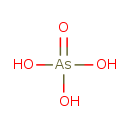
arsenate (PAMDB110239)
IUPAC:
hydrogen arsenate
CAS: 15584-04-0
Description: An arsenate ion resulting from the removal of two protons from arsenic acid.
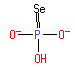
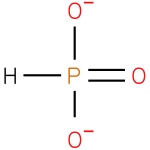
phosphonate (PAMDB110243)
IUPAC:
hydridotrioxidophosphate(2−)
CAS: Not Available
Description: A divalent inorganic anion obtained by removal of both protons from phosphonic acid