|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110214 |
|---|
|
Identification |
|---|
| Name: |
(R)-pantothenate |
|---|
| Description: | A pantothenate that is the conjugate base of (R)-pantothenic acid, obtained by deprotonation of the carboxy group. |
|---|
|
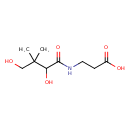
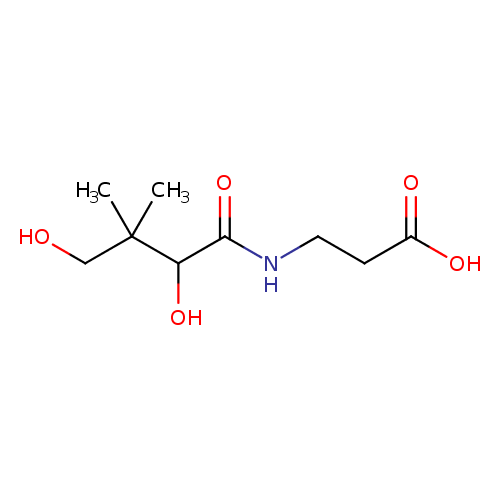
Structure |
|
|---|
| Synonyms: | -
vitamin B5
-
(R)-pantothenic acid
-
D-pantothenic acid
|
|---|
|
Chemical Formula: |
C9H16NO5
|
|---|
| Average Molecular Weight: |
218.23 |
|---|
| Monoisotopic Molecular
Weight: |
219.1106726614 |
|---|
| InChI Key: |
GHOKWGTUZJEAQD-ZETCQYMHSA-M |
|---|
| InChI: |
InChI=1S/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13)/p-1/t7-/m0/s1 |
|---|
| CAS
number: |
79-83-4 |
|---|
| IUPAC Name: | 3-[(2R)-2,4-dihydroxy-3,3-dimethylbutanamido]propanoate |
|---|
|
Traditional IUPAC Name: |
DL-pantothenic acid |
|---|
| SMILES: | CC(C)(CO)C(O)C(=O)NCCC(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Beta amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Beta amino acid or derivatives
- Fatty amide
- Monosaccharide
- N-acyl-amine
- Fatty acyl
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Organooxygen compound
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
< 25 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | < 25 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1000.0 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-3190000000-7a9e1fb7ca94ff4714e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00dl-9100000000-ada04a866c6d38235607 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0596-9000000000-aea14dc63426efd5a60d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [2026685 ]
- Loftus EV Jr, Tremaine WJ, Nelson RA, Shoemaker JD, Sandborn WJ, Phillips SF, Hasan Y: Dexpanthenol enemas in ulcerative colitis: a pilot study. Mayo Clin Proc. 1997 Jul;72(7):616-20. [9212762 ]
- Fry PC, Fox HM, Tao HG: Metabolic response to a pantothenic acid deficient diet in humans. J Nutr Sci Vitaminol (Tokyo). 1976;22(4):339-46. [1011047 ]
- Roth-Maier DA, Wauer A, Stangl GI, Kirchgessner M: Precaecal digestibility of niacin and pantothenic acid from different foods. Int J Vitam Nutr Res. 2000 Jan;70(1):8-13. [10683755 ]
- Preibisz J, Chlewicka I: [Digitalis treatment in acute myocardial infarct. Determination of serum drug levels] Pol Arch Med Wewn. 1977 Dec;58(6):585-91. [600836 ]
- Guilarte TR: A radiometric microbiological assay for pantothenic acid in biological fluids. Anal Biochem. 1989 Apr;178(1):63-6. [2499220 ]
- Srinivasan V, Christensen N, Wyse BW, Hansen RG: Pantothenic acid nutritional status in the elderly--institutionalized and noninstitutionalized. Am J Clin Nutr. 1981 Sep;34(9):1736-42. [7025609 ]
- Eissenstat BR, Wyse BW, Hansen RG: Pantothenic acid status of adolescents. Am J Clin Nutr. 1986 Dec;44(6):931-7. [3788840 ]
- Dastur DK, Santhadevi N, Quadros EV, Avari FC, Wadia NH, Desai MN, Bharucha EP: The B-vitamins in malnutrition with alcoholism. A model of intervitamin relationships. Br J Nutr. 1976 Sep;36(2):143-59. [182198 ]
|
|---|
| Synthesis Reference: |
Kataoka, Michihiko; Shimizu, Sakayu; Doi, Yukiko; Yamada, Hideaki. Stereospecific reduction of ethyl 2'-ketopantothenate to ethyl D-(+)-pantothenate with microbial cells as a catalyst. Applied and Environmental Microbiology (1990), 56(11), 3595-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|