Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 2311 - 2320 of
4373 in total
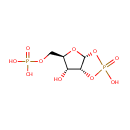
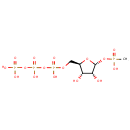
alpha-D-Ribose 1,2-cyclic phosphate 5-phosphate (PAMDB004852)
IUPAC:
{[(3aR,5R,6R,6aR)-2,6-dihydroxy-2-oxo-tetrahydro-2H-2???furo[2,3-d][1,3,2]dioxaphosphol-5-yl]methoxy}phosphonic acid
CAS: Not Available
Description: A ribose bisphosphate that is the cyclic-1,2-phosphate derivative of 5-phospho-α-D-ribose
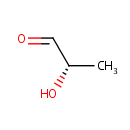
(S)-Lactaldehyde (PAMDB004860)
IUPAC:
(2S)-2-hydroxypropanal
CAS: 3913-64-2
Description: L-lactaldehyde is an intermediate metabolite in the pyruvate metabolism pathway. L-lactaldehyde is irreversibly produced from pyruvaldehyde via the enzyme aldehyde reductase (EC:1.1.1.21) which is then irreversibly converted to propylene glycol via aldehyde reductase (EC:1.1.1.21).
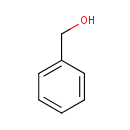
Benzyl alcohol (PAMDB005009)
IUPAC:
phenylmethanol
CAS: 100-51-6
Description: Benzyl Alcohol is a colorless liquid with a sharp burning taste and slight odor. It is used as a local anesthetic and to reduce pain associated with Lidocaine injection. Also, it is used in the manufacture of other benzyl compounds, as a pharmaceutical aid, and in perfumery and flavoring. Benzyl Alcohol is an aromatic alcohol used in a wide variety of cosmetic formulations as a fragrance component, preservative, solvent, and viscosity-decreasing agent. Benzyl Alcohol is metabolized to Benzoic Acid, which reacts with glycine and excreted as hippuric acid in the human body. Acceptable daily intakes were established by the World Health Organization at 5 mg/kg for Benzyl Alcohol. No adverse effects of benzyl alcohol have been seen in chronic exposure animal studies using rats and mice. Effects of Benzyl Alcohol in chronic exposure animal studies are limited to reduced feed intake and reduced growth. Some differences have been noted in one reproductive toxicity study using mice, but these were limited to lower maternal body weights and decreased mean litter weights. Another study also noted that fetal weight was decreased compared to controls, but a third study showed no differences between control and benzyl alcohol-treated groups. Benzyl Alcohol has been associated with an increased number of resorptions and malformations in hamsters, but there have been no reproductive or developmental toxicity findings in studies using mice and rats. Genotoxicity tests for benzyl alcohol are mostly negative, but there were some assays that were positive. Carcinogenicity studies, however, were negative. Clinical data indicates that benzyl alcohol can produce nonimmunologic contact urticaria and nonimmunologic immediate contact reactions, characterized by the appearance of wheals, erythema, and pruritis. 5% benzyl alcohol can elicit a reaction. Benzyl Alcohol is not a sensitizer at 10%. Benzyl Alcohol could be used safely at concentrations up to 5%, but that manufacturers should consider the nonimmunologic phenomena when using benzyl alcohol in cosmetic formulations designed for infants and children. Additionally, Benzyl Alcohol is considered safe up to 10% for use in hair dyes. The limited body exposure, the duration of use, and the frequency of use are considered in concluding that the nonimmunologic reactions would not be a concern. Because of the wide variety of product types in which benzyl alcohol may be used, it is likely that inhalation may be a route of exposure. The available safety tests are not considered sufficient to support the safety of benzyl alcohol in formulations where inhalation is a route of exposure. Inhalation toxicity data are needed to complete the safety assessment of benzyl alcohol where inhalation can occur. .
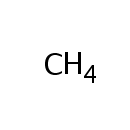
Methane (PAMDB005079)
IUPAC:
methane
CAS: 74-82-8
Description: A one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms. It is a colourless, odourless, non-toxic but flammable gas (b.p. ??61?C).
Lipid IIA (PAMDB005112)
IUPAC:
{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-6-[({[(2R,3R,4S,5S)-5-amino-3,4-dihydroxyoxan-2-yl]oxy}(hydroxy)phosphoryl)oxy]-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-2-yl]methoxy}-2-(hydroxymethyl)-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-3-yl]oxy}phosphonic acid
CAS: Not Available
Description: A lipid A derivative in which the phospho group at the anomeric carbon is esterified with a 4-amino-4-deoxy-β-L-arabinopyranosyl group
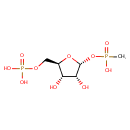
alpha-D-Ribose 1-methylphosphonate 5-triphosphate (PAMDB005128)
IUPAC:
({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-{[hydroxy(methyl)phosphoryl]oxy}oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid
CAS: Not Available
Description: In Pseudomonas aeruginosa, alpha-D-Ribose 1-methylphosphonate 5-triphosphate is an intermediate in the transformation of Phosphonates to Phosphate. The enzyme alpha-D-ribose 1-methylphosphonate 5-triphosphate synthase (EC 2.7.8.37) catalyses the reaction ATP + methylphosphonate <=> alpha-D-ribose 1-methylphosphonate 5-triphosphate + adenine .
alpha-D-Ribose 1-methylphosphonate 5-phosphate (PAMDB005129)
IUPAC:
{[(2R,3S,4R,5R)-3,4-dihydroxy-5-{[hydroxy(methyl)phosphoryl]oxy}oxolan-2-yl]methoxy}phosphonic acid
CAS: Not Available
Description: An organophosphate oxoanion obtained by deprotonation of the phosphate and phosphonate OH groups of α-D-ribose 1-methylphosphonate 5-phosphate; major species at pH 7.3.
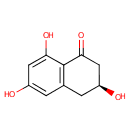
tRNA(Tyr) (PAMDB005478)
IUPAC:
(3S)-3,6,8-trihydroxy-1,2,3,4-tetrahydronaphthalen-1-one
CAS: Not Available
Description: Non-coding transfer RNAs
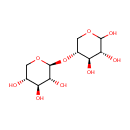
tRNA(Ala) (PAMDB005597)
IUPAC:
(3R,4R,5R)-5-{[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}oxane-2,3,4-triol
CAS: Not Available
Description: Non-coding transfer RNAs
L-Ornithine (PAMDB005689)
IUPAC:
(2S)-2,5-diaminopentanoic acid
CAS: 70-26-8
Description: An optically active form of ornithine having L-configuration