|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB005597 |
|---|
|
Identification |
|---|
| Name: |
tRNA(Ala) |
|---|
| Description: | Non-coding transfer RNAs |
|---|
|
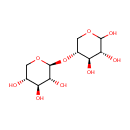
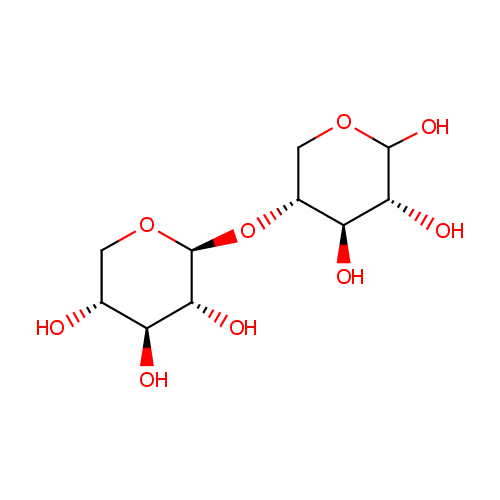
Structure |
|
|---|
| Synonyms: | - (1,4)-b-Xylobiose
- (1,4)-beta-Xylobiose
- (1,4)-β-xylobiose
- (Xyl)2
- 1,4-b-Xylobiose
- 1,4-beta-Xylobiose
- 1,4-β-xylobiose
- 4-O-b-D-Xylopyranosyl-D-xylopyranose
- 4-O-b-D-Xylopyranosyl-D-xylose
- 4-O-beta-D-Xylopyranosyl-D-xylopyranose
- 4-O-beta-D-Xylopyranosyl-D-xylose
- 4-O-β-D-xylopyranosyl-D-xylopyranose
- 4-O-β-D-xylopyranosyl-D-xylose
- b-D-Xyl-(1->4)-D-xyl
- b-D-Xylp-(1->4)-D-xylp
- beta-D-Xyl-(1->4)-D-xyl
- beta-D-Xylp-(1->4)-D-xylp
- Xylbeta(1,4)xyl
- Xylbeta(1->4)xyl
- β-D-xyl-(1->4)-D-xyl
- β-D-xylp-(1->4)-D-xylp
|
|---|
|
Chemical Formula: |
C10H18O9 |
|---|
| Average Molecular Weight: |
282.245 |
|---|
| Monoisotopic Molecular
Weight: |
282.09508216 |
|---|
| InChI Key: |
LGQKSQQRKHFMLI-WSNPFVOISA-N |
|---|
| InChI: | InChI=1S/C10H18O9/c11-3-1-18-10(8(15)5(3)12)19-4-2-17-9(16)7(14)6(4)13/h3-16H,1-2H2/t3-,4-,5+,6+,7-,8-,9?,10+/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3R,4R,5R)-5-{[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}oxane-2,3,4-triol |
|---|
|
Traditional IUPAC Name: |
(3R,4R,5R)-5-{[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}oxane-2,3,4-triol |
|---|
| SMILES: | O[C@@H]1CO[C@@H](O[C@@H]2COC(O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Glycosyl compounds |
|---|
|
Direct Parent |
O-glycosyl compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- O-glycosyl compound
- Disaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|