|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120595 |
|---|
|
Identification |
|---|
| Name: |
pseudouridine |
|---|
| Description: | A C-glycosyl pyrimidine that consists of uracil having a β-D-ribofuranosyl residue attached at position 5. The C-glycosyl isomer of the nucleoside uridine. |
|---|
|
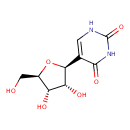
Structure |
|
|---|
| Synonyms: | - (1S)-1,4-anhydro-1-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-D-ribitol
- 5-(β-D-ribofuranosyl)uracil
- β-Pseudouridine
- p
- Pseudouridine
- pseudouridine
- Ψ-uridine
|
|---|
|
Chemical Formula: |
C9H12N2O6 |
|---|
| Average Molecular Weight: |
244.204 |
|---|
| Monoisotopic Molecular
Weight: |
244.06953 |
|---|
| InChI Key: |
PTJWIQPHWPFNBW-GBNDHIKLSA-N |
|---|
| InChI: | InChI=1S/C9H12N2O6/c12-2-4-5(13)6(14)7(17-4)3-1-10-9(16)11-8(3)15/h1,4-7,12-14H,2H2,(H2,10,11,15,16)/t4-,5-,6-,7+/m1/s1 |
|---|
| CAS
number: |
1445-07-4 |
|---|
| IUPAC Name: | 5-(β-D-ribofuranosyl)pyrimidine-2,4(1H,3H)-dione |
|---|
|
Traditional IUPAC Name: |
?-pseudouridine |
|---|
| SMILES: | C1(=C(C(=O)NC(=O)N1)C2(OC(CO)C(O)C(O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as nucleoside and nucleotide analogues. These are analogues of nucleosides and nucleotides. These include phosphonated nucleosides, C-glycosylated nucleoside bases, analogues where the sugar unit is a pyranose, and carbocyclic nucleosides, among others. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Nucleoside and nucleotide analogues |
|---|
|
Direct Parent |
Nucleoside and nucleotide analogues |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- C-glycosyl compound
- Glycosyl compound
- Pentose monosaccharide
- Pyrimidone
- Hydropyrimidine
- Monosaccharide
- Pyrimidine
- Oxolane
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Secondary alcohol
- Urea
- Azacycle
- Oxacycle
- Ether
- Dialkyl ether
- Organoheterocyclic compound
- Primary alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Hu Y, Tang Q, Liu B (1997)[Determination of pseudouridine in serum by high performance liquid chromatography]. Se pu = Chinese journal of chromatography 15, Pubmed: 15739475
- COHN WE (1960)Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. The Journal of biological chemistry 235, Pubmed: 13811056
- Badis G, Fromont-Racine M, Jacquier A (2003)A snoRNA that guides the two most conserved pseudouridine modifications within rRNA confers a growth advantage in yeast. RNA (New York, N.Y.) 9, Pubmed: 12810910
- Patton JR, Padgett RW (2005)Pseudouridine modification in Caenorhabditis elegans spliceosomal snRNAs: unique modifications are found in regions involved in snRNA-snRNA interactions. BMC molecular biology 6, Pubmed: 16236171
- Kiss AM, Jády BE, Bertrand E, Kiss T (2004)Human box H/ACA pseudouridylation guide RNA machinery. Molecular and cellular biology 24, Pubmed: 15199136
- Durairaj A, Limbach PA (2008)Mass spectrometry of the fifth nucleoside: a review of the identification of pseudouridine in nucleic acids. Analytica chimica acta 623, Pubmed: 18620915
- Blaby IK, Majumder M, Chatterjee K, Jana S, Grosjean H, de Crécy-Lagard V, Gupta R (2011)Pseudouridine formation in archaeal RNAs: The case of Haloferax volcanii. RNA (New York, N.Y.) 17, Pubmed: 21628430
- Addepalli B, Limbach PA (2011)Mass spectrometry-based quantification of pseudouridine in RNA. Journal of the American Society for Mass Spectrometry 22, Pubmed: 21953190
- Roux A, Xu Y, Heilier JF, Olivier MF, Ezan E, Tabet JC, Junot C (2012)Annotation of the human adult urinary metabolome and metabolite identification using ultra high performance liquid chromatography coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer. Analytical chemistry 84, Pubmed: 22770225
- Taucher M, Ganisl B, Breuker K (2011)Identification, localization, and relative quantitation of pseudouridine in RNA by tandem mass spectrometry of hydrolysis products. International journal of mass spectrometry 304, Pubmed: 21960742
|
|---|
| Synthesis Reference: |
anessian, Stephen; Machaalani, Roger. A highly stereo-controlled and efficient synthesis of a- and b-pseudouridines. Tetrahedron Letters (2003), 44(45), 8321-8323. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|