|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120479 |
|---|
|
Identification |
|---|
| Name: |
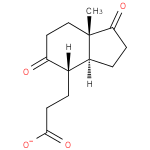
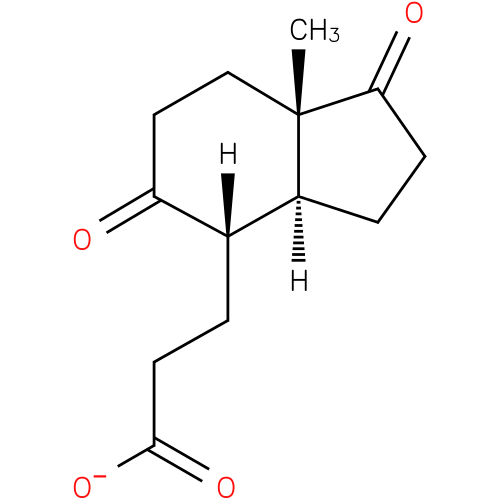
3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate |
|---|
| Description: | A dioxo monocarboxylic acid anion that is the conjugate base of 9,17-dioxo-1,2,3,4,10,19-hexanorandrostan-5-oic acid, arising from deprotonation of the carboxy group; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate
- 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydroinden-4-yl]propanoate
- 9,17-dioxo-1,2,3,4,10,19-hexanorandrostan-5-oate(1−)
|
|---|
|
Chemical Formula: |
C13H17O4 |
|---|
| Average Molecular Weight: |
237.275 |
|---|
| Monoisotopic Molecular
Weight: |
238.12051 |
|---|
| InChI Key: |
PCCFNLPWOFTZPJ-RVBZMBCESA-M |
|---|
| InChI: | InChI=1S/C13H18O4/c1-13-7-6-10(14)8(2-5-12(16)17)9(13)3-4-11(13)15/h8-9H,2-7H2,1H3,(H,16,17)/p-1/t8-,9-,13-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC12(CCC(=O)C(CCC(=O)[O-])[CH](CCC(=O)1)2) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as carbocyclic fatty acids. These are fatty acids containing a carbocyclic ring . |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Carbocyclic fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Carbocyclic fatty acid
- Cyclic ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Organic anion
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
- a small molecule (CPD-13711)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | (1E,2Z)-3-hydroxy-5,9,17-trioxo-4,5:9,10-disecoandrosta-1 (10),2-dien-4-oate + Water → 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate + (2Z,4Z)-2-hydroxyhexa-2,4-dienoate + Hydrogen ion3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate + ATP + coenzyme A → 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoyl-CoA + AMP + diphosphate |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Horinouchi M, Hayashi T, Koshino H, Kurita T, Kudo T (2005)Identification of 9,17-dioxo-1,2,3,4,10,19-hexanorandrostan-5-oic acid, 4-hydroxy-2-oxohexanoic acid, and 2-hydroxyhexa-2,4-dienoic acid and related enzymes involved in testosterone degradation in Comamonas testosteroni TA441. Applied and environmental microbiology 71, Pubmed: 16151114
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|