|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120442 |
|---|
|
Identification |
|---|
| Name: |
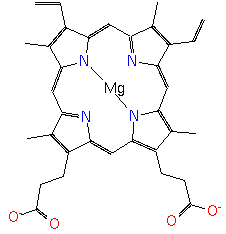
Mg-protoporphyrin |
|---|
| Description: | The conjugate base of magnesium protoporphyrin, formed by deprotonation of the carboxyethyl groups at C-13 and C-17. It is the principal species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Mg-protoporphyrin IX
- magnesium protoporphyrin
- MgP
|
|---|
|
Chemical Formula: |
C34H30N4O4MG |
|---|
| Average Molecular Weight: |
582.94 |
|---|
| Monoisotopic Molecular
Weight: |
584.2274 |
|---|
| InChI Key: |
REJJDEGSUOCEEW-RGGAHWMASA-J |
|---|
| InChI: | InChI=1S/C34H34N4O4.Mg/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-4/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16-; |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | Not Available |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C=CC1(C5(N6(C(C=1C)=CC2(C(=C(C(N=2)=CC3(N(C(=C(C=3CCC([O-])=O)C)C=C4(C(=C(C(=N4)C=5)C)C=C))[Mg]6))CCC(=O)[O-])C)))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as metalloporphyrins. These are polycyclic compounds containing a porphyrin moiety and a metal atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Metallotetrapyrroles |
|---|
|
Direct Parent |
Metalloporphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Metalloporphyrin
- Porphyrin
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Carboxylic acid salt
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organic salt
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
| Property | Value | Source |
|---|
| Molecular Weight | 582.943 g/mol | PubChem | | Hydrogen Bond Donor Count | 0 | PubChem | | Hydrogen Bond Acceptor Count | 8 | PubChem | | Rotatable Bond Count | 6 | PubChem | | Exact Mass | 582.212 g/mol | PubChem | | Monoisotopic Mass | 582.212 g/mol | PubChem | | Topological Polar Surface Area | 107 A^2 | PubChem | | Heavy Atom Count | 43 | PubChem | | Formal Charge | -2 | PubChem | | Complexity | 1570 | PubChem | | Isotope Atom Count | 0 | PubChem | | Defined Atom Stereocenter Count | 0 | PubChem | | Undefined Atom Stereocenter Count | 0 | PubChem | | Defined Bond Stereocenter Count | 0 | PubChem | | Undefined Bond Stereocenter Count | 0 | PubChem | | Covalently-Bonded Unit Count | 2 | PubChem |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- chlorophyllide a biosynthesis III (aerobic, light independent)PWY-7159
- chlorophyllide a biosynthesis I (aerobic, light-dependent)CHLOROPHYLL-SYN
- chlorophyllide a biosynthesis II (anaerobic)PWY-5531
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|