|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120437 |
|---|
|
Identification |
|---|
| Name: |
vanillate |
|---|
| Description: | A methoxybenzoate that is the conjugate base of vanillic acid. |
|---|
|
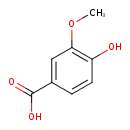
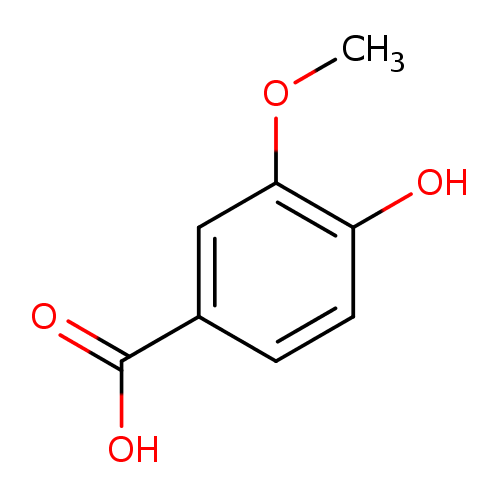
Structure |
|
|---|
| Synonyms: | - 4-HYDROXY-3-METHOXYBENZOATE
- vanillate
|
|---|
|
Chemical Formula: |
C8H7O4 |
|---|
| Average Molecular Weight: |
167.141 |
|---|
| Monoisotopic Molecular
Weight: |
168.04225 |
|---|
| InChI Key: |
WKOLLVMJNQIZCI-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C8H8O4/c1-12-7-4-5(8(10)11)2-3-6(7)9/h2-4,9H,1H3,(H,10,11)/p-1 |
|---|
| CAS
number: |
121-34-6 |
|---|
| IUPAC Name: | 4-hydroxy-3-methoxybenzoate |
|---|
|
Traditional IUPAC Name: |
vanillic acid |
|---|
| SMILES: | COC1(=CC(=CC=C(O)1)C(=O)[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as m-methoxybenzoic acids and derivatives. These are benzoic acids in which the hydrogen atom at position 3 of the benzene ring is replaced by a methoxy group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
M-methoxybenzoic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- M-methoxybenzoic acid or derivatives
- Hydroxybenzoic acid
- Methoxyphenol
- Benzoic acid
- Anisole
- Phenoxy compound
- Benzoyl
- Phenol ether
- Methoxybenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Monocarboxylic acid or derivatives
- Ether
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
211.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 211.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1.5 mg/mL at 14 °C | YALKOWSKY,SH & DANNENFELSER,RM (1992) | | LogP | 1.43 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-0hp2-2691000000-8409d9c758b023c6576b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0gb9-0900000000-0aa6709c16899e222086 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0a4i-0900000000-9aa2b9d4aa89e6ac12ab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0a4i-0900000000-6d98ebfa89f4b8ee1e6b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-0gb9-2900000000-babed327a6e49c6f7d51 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-105f33777cee542cf6df | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-05fr-1900000000-a502792703fa2f211035 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0a4l-5900000000-037ef32f4c68a5252b92 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0a4i-9800000000-ecd60ac022f5fee1d5ef | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-066u-9300000000-c63f7f792794f3c748a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0gb9-6900000000-c13506201c3612fc7ca5 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Muskiet FA, Groen A: Urinary excretion of conjugated homovanillic acid, 3,4-dihydroxyphenylacetic acid, p-hydroxyphenylacetic acid, and vanillic acid by persons on their usual diet and patients with neuroblastoma. Clin Chem. 1979 Jul;25(7):1281-4. [455649 ]
- Ebinger G, Verheyden R: On the occurence of vanillic acid in human brain and cerebrospinal fluid. J Neurol. 1976 Apr 23;212(2):133-8. [57225 ]
- Lee BL, New AL, Kok PW, Ong HY, Shi CY, Ong CN: Urinary trans,trans-muconic acid determined by liquid chromatography: application in biological monitoring of benzene exposure. Clin Chem. 1993 Sep;39(9):1788-92. [8375048 ]
- Dhananjaya BL, Nataraju A, Rajesh R, Raghavendra Gowda CD, Sharath BK, Vishwanath BS, D'Souza CJ: Anticoagulant effect of Naja naja venom 5'nucleotidase: demonstration through the use of novel specific inhibitor, vanillic acid. Toxicon. 2006 Sep 15;48(4):411-21. Epub 2006 Jul 7. [16899266 ]
|
|---|
| Synthesis Reference: |
Pearl, Irwin A. Reactions of vanillin and its derived compounds. I. The reaction of vanillin with silver oxide. Journal of the American Chemical Society (1946), 68 429-32. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|