|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120327 |
|---|
|
Identification |
|---|
| Name: |
2-oxo-4-methylthiobutanoate |
|---|
| Description: | The 2-oxo monocarboxylic acid anion derived from 4-methylthio-2-oxobutanoic acid. The major microspecies at pH 7.3, it is formed from L-methionine via the action of methionine transaminase. |
|---|
|
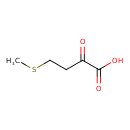
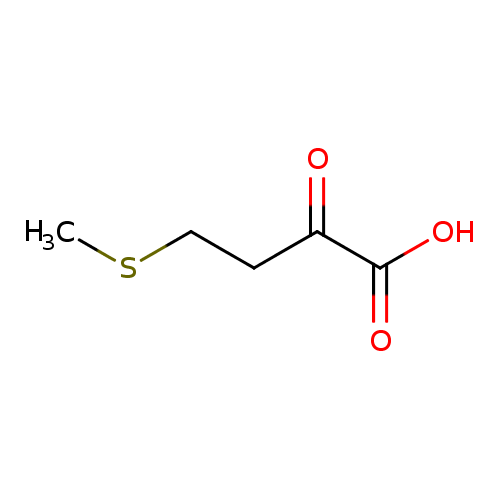
Structure |
|
|---|
| Synonyms: | - 2-oxo-4-methylthiobutanoate
- 4-methylthio-2-oxobutanoate
|
|---|
|
Chemical Formula: |
C5H7O3S |
|---|
| Average Molecular Weight: |
147.168 |
|---|
| Monoisotopic Molecular
Weight: |
148.01941 |
|---|
| InChI Key: |
SXFSQZDSUWACKX-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C5H8O3S/c1-9-3-2-4(6)5(7)8/h2-3H2,1H3,(H,7,8)/p-1 |
|---|
| CAS
number: |
583-92-6 |
|---|
| IUPAC Name: | 4-(methylsulfanyl)-2-oxobutanoate |
|---|
|
Traditional IUPAC Name: |
2-oxo-4-thiomethylbutyric acid |
|---|
| SMILES: | CSCCC(C([O-])=O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as thia fatty acids. These are fatty acid derivatives obtained by insertion of a sulfur atom at specific positions in the chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Thia fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Short-chain keto acid
- Thia fatty acid
- Alpha-keto acid
- Keto acid
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Dialkylthioether
- Monocarboxylic acid or derivatives
- Sulfenyl compound
- Thioether
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Heilbronn J, Wilson J, Berger BJ (1999)Tyrosine aminotransferase catalyzes the final step of methionine recycling in Klebsiella pneumoniae. Journal of bacteriology 181, Pubmed: 10074065
- Dolzan M, Johansson K, Roig-Zamboni V, Campanacci V, Tegoni M, Schneider G, Cambillau C (2004)Crystal structure and reactivity of YbdL from Escherichia coli identify a methionine aminotransferase function. FEBS letters 571, Pubmed: 15280032
|
|---|
| Synthesis Reference: |
Brodelius, P.; Hagerdal, B.; Mosbach, K. Immobilized whole cells of the yeast Trigonopsis variabilis containing D-amino acid oxidase for the production of a-keto acids. Enzyme Engineering (1980), 5 383-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|