|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110838 |
|---|
|
Identification |
|---|
| Name: |
N5-methyl-tetrahydropteroyl mono-L-glutamate |
|---|
| Description: | 5 methyltetrahydrofolic acid (5-MTHF) is the most biologically active form of the B-vitamin known as folic acid, also known generically as folate. 5-MTHF functions, in concert with vitamin B12, as a methyl-group donor involved in the conversion of the amino acid homocysteine to methionine. Methyl (CH3) group donation is vital to many bodily processes, including serotonin, melatonin, and DNA synthesis. Therapeutically, 5-MTHF is instrumental in reducing homocysteine levels, preventing neural tube defects, and improving vascular endothelial function. Research on folate supplementation suggests it plays a key role in preventing cervical dysplasia and protecting against neoplasia in ulcerative colitis. Folic acid also shows promise as part of a nutritional protocol to treat vitiligo, and may reduce inflammation of the gingiva. Furthermore, certain neurological, cognitive, and psychiatric presentations may be secondary to folate deficiency. Such presentations include depression, peripheral neuropathy, myelopathy, restless legs syndrome, insomnia, dementia, forgetfulness, irritability, endogenous depression, organic psychosis, and schizophrenia-like syndromes. After ingestion, the process of conversion of folic acid to the metabolically active coenzyme forms is relatively complex. Synthesis of the active forms of folic acid requires several enzymes, adequate liver and intestinal function, and adequate supplies of riboflavin (B2), niacin (B3), pyridoxine (B6), zinc, vitamin C, and serine. After formation of the coenzyme forms of the vitamin in the liver, these metabolically active compounds are secreted into the small intestine with bile (the folate enterohepatic cycle), where they are reabsorbed and distributed to tissues throughout the body. Human pharmacokinetic studies indicate folic acid has high bioavailability, with large oral doses of folic acid substantially raising plasma levels in healthy subjects in a time and dose dependent manner. Red blood cells (RBCs) appear to be the storage depot for folic acid, as RBC levels remain elevated for periods in excess of 40 days following discontinuation of supplementation. Folic acid is poorly transported to the brain and rapidly cleared from the central nervous system. The primary methods of elimination of absorbed folic acid are fecal (through bile) and urinary. Despite the biochemical complexity of this process, evidence suggests oral supplementation with folic acid increases the body's pool of 5-MTHF in healthy individuals. However, enzyme defects, mal-absorption, digestive system pathology, and liver disease can result in impaired ability to activate folic acid. In fact, some individuals have a severe congenital deficiency of the enzyme Methyl tetrahydrofolate reductase (5-MTHFR), which is needed to convert folic acid to 5-MTHF. Milder forms of this enzyme defect likely interact with dietary folate status to determine risk for some disease conditions. In individuals with a genetic defect of this enzyme (whether mild or severe), supplementation with 5- MTHF might be preferable to folic acid supplementation. (PMID: 17176169 ). |
|---|
|
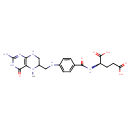
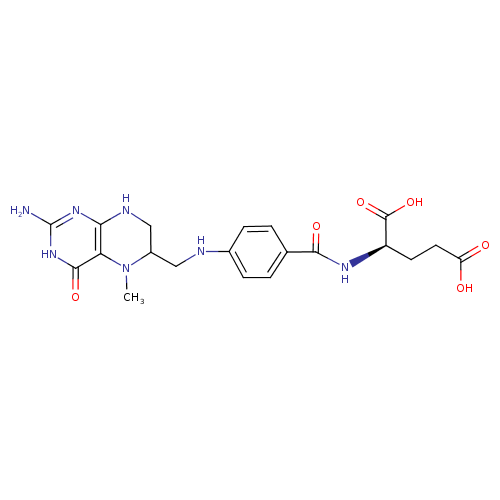
Structure |
|
|---|
| Synonyms: | -
5-methyl-tetrahydrofolate mono-L-glutamate
-
5-methyl-THF mono-L-glutamate
-
5-methyl-5,6,7,8-tetrahydrofolate mono-L-glutamate
-
n5-methyltetrahydrofolate mono-L-glutamate
-
N5-methyl-THF mono-L-glutamate
-
methyl-THF mono-L-glutamate
-
methyl-tetrahydrofolate mono-L-glutamate
-
methyl-H4F mono-L-glutamate
-
5-methyl-5,6,7,8-tetrahydropteroyl-L-glutamate
-
N5-methyl--H4PteGlu1
|
|---|
|
Chemical Formula: |
C20H23N7O6
|
|---|
| Average Molecular Weight: |
457.44 |
|---|
| Monoisotopic Molecular
Weight: |
459.1866315715 |
|---|
| InChI Key: |
ZNOVTXRBGFNYRX-STQMWFEESA-L |
|---|
| InChI: |
InChI=1S/C20H25N7O6/c1-27-12(9-23-16-15(27)18(31)26-20(21)25-16)8-22-11-4-2-10(3-5-11)17(30)24-13(19(32)33)6-7-14(28)29/h2-5,12-13,22H,6-9H2,1H3,(H,24,30)(H,28,29)(H,32,33)(H4,21,23,25,26,31)/p-2/t12-,13-/m0/s1 |
|---|
| CAS
number: |
134-35-0 |
|---|
| IUPAC Name: | (2S)- 2- 2- {[4- {[4- ({[(6S)- ({[(6S)- 2- 2- amino- amino- 5- 5- methyl- methyl- 4- 4- oxo- oxo- 3,4,5,6,7,8- 3,4,5,6,7,8- hexahydropteridin- hexahydropteridin- 6- 6- yl]methyl}amino)benzoyl]amino}pentanedioate yl]methyl}amino)benzoyl]amino}pentanedioate |
|---|
|
Traditional IUPAC Name: |
(2R)-2-[(4-{[(2-amino-5-methyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| SMILES: | CN2([CH](CNC1(=C(C(=O)NC(N)=N1)2))CNC3(C=CC(C(=O)NC(C(=O)[O-])CCC([O-])=O)=CC=3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Tetrahydrofolic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrahydrofolic acid
- Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Dialkylarylamine
- Benzoyl
- Tertiary aliphatic/aromatic amine
- Aniline or substituted anilines
- Phenylalkylamine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Aminopyrimidine
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Benzenoid
- Primary aromatic amine
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Tertiary amine
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Organic oxide
- Primary amine
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Van Hove JL, Lazeyras F, Zeisel SH, Bottiglieri T, Hyland K, Charles HC, Gray L, Jaeken J, Kahler SG: One-methyl group metabolism in non-ketotic hyperglycinaemia: mildly elevated cerebrospinal fluid homocysteine levels. J Inherit Metab Dis. 1998 Dec;21(8):799-811. [9870205 ]
- Kane MA, Roth E, Raptis G, Schreiber C, Waxman S: Effect of intracellular folate concentration on the modulation of 5-fluorouracil cytotoxicity by the elevation of phosphoribosylpyrophosphate in cultured human KB cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6444-50. [2445472 ]
- Garbis SD, Melse-Boonstra A, West CE, van Breemen RB: Determination of folates in human plasma using hydrophilic interaction chromatography-tandem mass spectrometry. Anal Chem. 2001 Nov 15;73(22):5358-64. [11816560 ]
- Ormazabal A, Artuch R, Vilaseca MA, Aracil A, Pineda M: Cerebrospinal fluid concentrations of folate, biogenic amines and pterins in Rett syndrome: treatment with folinic acid. Neuropediatrics. 2005 Dec;36(6):380-5. [16429378 ]
- Chladek J, Sispera L, Martinkova J: High-performance liquid chromatographic assay for the determination of 5-methyltetrahydrofolate in human plasma. J Chromatogr B Biomed Sci Appl. 2000 Jul 21;744(2):307-13. [10993519 ]
- Prasad PD, Mahesh VB, Leibach FH, Ganapathy V: Functional coupling between a bafilomycin A1-sensitive proton pump and a probenecid-sensitive folate transporter in human placental choriocarcinoma cells. Biochim Biophys Acta. 1994 Jun 30;1222(2):309-14. [8031869 ]
- Kim TH, Yang J, Darling PB, O'Connor DL: A large pool of available folate exists in the large intestine of human infants and piglets. J Nutr. 2004 Jun;134(6):1389-94. [15173401 ]
- Camilo E, Zimmerman J, Mason JB, Golner B, Russell R, Selhub J, Rosenberg IH: Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology. 1996 Apr;110(4):991-8. [8613033 ]
- Kamen BA, Smith AK: A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004 Apr 29;56(8):1085-97. [15094208 ]
- Surtees R, Leonard J, Austin S: Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991 Dec 21-28;338(8782-8783):1550-4. [1683972 ]
- Surtees R, Hyland K, Smith I: Central-nervous-system methyl-group metabolism in children with neurological complications of HIV infection. Lancet. 1990 Mar 17;335(8690):619-21. [1969014 ]
- Evans MI, Duquette DA, Rinaldo P, Bawle E, Rosenblatt DS, Whitty J, Quintero RA, Johnson MP: Modulation of B12 dosage and response in fetal treatment of methylmalonic aciduria (MMA): titration of treatment dose to serum and urine MMA. Fetal Diagn Ther. 1997 Jan-Feb;12(1):21-3. [9101216 ]
- Irizarry MC: Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004 Apr;1(2):226-34. [15717023 ]
- Straw JA, Szapary D, Wynn WT: Pharmacokinetics of the diastereoisomers of leucovorin after intravenous and oral administration to normal subjects. Cancer Res. 1984 Jul;44(7):3114-9. [6609768 ]
- 5-methyltetrahydrofolate. Monograph. Altern Med Rev. 2006 Dec;11(4):330-7. [17176169 ]
|
|---|
| Synthesis Reference: |
Gennari, Federico. Process for producing 5-methyltetrahydrofolic acid and its salts. U.S. (1992), 6 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|