|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110803 |
|---|
|
Identification |
|---|
| Name: |
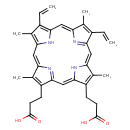
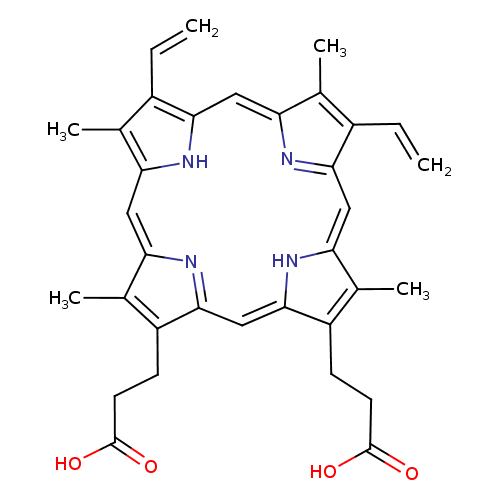
protoporphyrin IX |
|---|
| Description: | The dicarboxylate anion of protoporphyrin, obtained by deprotonation of both carboxy groups. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C34H32N4O4
|
|---|
| Average Molecular Weight: |
560.65 |
|---|
| Monoisotopic Molecular
Weight: |
562.2580056006 |
|---|
| InChI Key: |
KSFOVUSSGSKXFI-UJJXFSCMSA-L |
|---|
| InChI: |
InChI=1S/C34H34N4O4/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25/h7-8,13-16,35,38H,1-2,9-12H2,3-6H3,(H,39,40)(H,41,42)/p-2/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- |
|---|
| CAS
number: |
553-12-8 |
|---|
| IUPAC Name: | 7,12-diethenyl-3,8,13,17-tetramethylporphyrin-2,18-dipropanoate |
|---|
|
Traditional IUPAC Name: |
protoporphyrin |
|---|
| SMILES: | C=CC1(C(C)=C2(C=C5(C(C)=C(CCC([O-])=O)C(C=C4(C(CCC([O-])=O)=C(C)C(=CC3(C(C=C)=C(C)C(=CC=1N2)N=3))N4))=N5))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Dicarboxylic acid or derivatives
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
- a porphyrin (PROTOPORPHYRIN_IX)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
300 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 300 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.169 mg/mL at 25 °C | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Messmann H, Knuchel R, Baumler W, Holstege A, Scholmerich J: Endoscopic fluorescence detection of dysplasia in patients with Barrett's esophagus, ulcerative colitis, or adenomatous polyps after 5-aminolevulinic acid-induced protoporphyrin IX sensitization. Gastrointest Endosc. 1999 Jan;49(1):97-101. [9869731 ]
- Stankiewicz A, Lutz W, Krajewska B, Szulc B: [Plasma indicators of iron metabolism in persons occupationally exposed to organic solvents with normal and increased levels of protoporphyrin IX in erythrocytes] Pol Tyg Lek. 1986 Jul 7;41(27):851-4. [3763462 ]
- Stankiewicz A: [Erythrocyte protoporphyrin IX in occupational exposure to asbestos] Med Pr. 1984;35(5):351-4. [6530952 ]
- Sudworth CD, Stringer MR, Cruse-Sawyer JE, Brown SB: Fluorescence microspectroscopy technique for the study of intracellular protoporphyrin IX dynamics. Appl Spectrosc. 2003 Jun;57(6):682-8. [14658702 ]
- Kufner G, Schlegel H, Jager R: A spectrophotometric micromethod for determining erythrocyte protoporphyrin-IX in whole blood or erythrocytes. Clin Chem Lab Med. 2005;43(2):183-91. [15843214 ]
- Stankiewicz A: The concentration of protoporphyrin IX in workers occupationally exposed to lead. Mater Med Pol. 1989 Apr-Jun;21(2):100-2. [2488459 ]
- Krieg RC, Fickweiler S, Wolfbeis OS, Knuechel R: Cell-type specific protoporphyrin IX metabolism in human bladder cancer in vitro. Photochem Photobiol. 2000 Aug;72(2):226-33. [10946577 ]
- Bailey GG, Needham LL: Simultaneous quantification of erythrocyte zinc protoporphyrin and protoporphyrin IX by liquid chromatography. Clin Chem. 1986 Dec;32(12):2137-42. [3779978 ]
- Chisolm J Jr, Brown DH: Micro-scale photofluorometric determination of "free erythrocyte pophyrin" (protoporphyrin IX). Clin Chem. 1975 Oct;21(11):1669-82. [1164799 ]
- Casas A, Batlle AM, Butler AR, Robertson D, Brown EH, MacRobert A, Riley PA: Comparative effect of ALA derivatives on protoporphyrin IX production in human and rat skin organ cultures. Br J Cancer. 1999 Jul;80(10):1525-32. [10408393 ]
- Smits T, Robles CA, van Erp PE, van de Kerkhof PC, Gerritsen MJ: Correlation between macroscopic fluorescence and protoporphyrin IX content in psoriasis and actinic keratosis following application of aminolevulinic acid. J Invest Dermatol. 2005 Oct;125(4):833-9. [16185285 ]
- Bartosova J, Hrkal Z: Accumulation of protoporphyrin-IX (PpIX) in leukemic cell lines following induction by 5-aminolevulinic acid (ALA). Comp Biochem Physiol C Toxicol Pharmacol. 2000 Jul;126(3):245-52. [11048674 ]
- Sakai T, Takeuchi Y, Ikeya Y, Araki T, Ushio K: [Automated HPLC method for determining zinc protoporphyrin IX and protoporphyrin IX in erythrocytes of workers exposed to lead] Sangyo Igaku. 1988 Nov;30(6):467-74. [3221502 ]
- Stankiewicz A, Lutz W, Szeszko A: [Protoporphyrin IX level in erythrocytes of persons with alcoholic liver cirrhosis] Pol Tyg Lek. 1985 Jul 15;40(28):787-9. [4059100 ]
- Star WM, Aalders MC, Sac A, Sterenborg HJ: Quantitative model calculation of the time-dependent protoporphyrin IX concentration in normal human epidermis after delivery of ALA by passive topical application or lontophoresis. Photochem Photobiol. 2002 Apr;75(4):424-32. [12003134 ]
- von Beckerath M, Juzenas P, Ma LW, Iani V, Lofgren L, Moan J: The influence of UV exposure on 5-aminolevulinic acid-induced protoporphyrin IX production in skin. Photochem Photobiol. 2001 Dec;74(6):825-8. [11783939 ]
- Gottsch JD, Graham CR Jr, Hairston RJ, Chen CH, Green WR, Stark WJ: Protoporphyrin IX photosensitization of corneal endothelium. Arch Ophthalmol. 1989 Oct;107(10):1497-500. [2803100 ]
- Bissonnette R, Zeng H, McLean DI, Korbelik M, Lui H: Oral aminolevulinic acid induces protoporphyrin IX fluorescence in psoriatic plaques and peripheral blood cells. Photochem Photobiol. 2001 Aug;74(2):339-45. [11547574 ]
- Rick K, Sroka R, Stepp H, Kriegmair M, Huber RM, Jacob K, Baumgartner R: Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. J Photochem Photobiol B. 1997 Oct;40(3):313-9. [9372622 ]
- De Rosa FS, Marchetti JM, Thomazini JA, Tedesco AC, Bentley MV: A vehicle for photodynamic therapy of skin cancer: influence of dimethylsulphoxide on 5-aminolevulinic acid in vitro cutaneous permeation and in vivo protoporphyrin IX accumulation determined by confocal microscopy. J Control Release. 2000 Apr 3;65(3):359-66. [10699294 ]
|
|---|
| Synthesis Reference: |
Games, David E.; Jackson, Anthony H.; Jackson, J. Richard; Belcher, Roderick V.; Smith, Sydney G. Biosynthesis of protoporphyrin-IX from coproporphyrinogen-III. Journal of the Chemical Society, Chemical Communications (1976), (6), 187-8. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|