|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110034 |
|---|
|
Identification |
|---|
| Name: |
thiamin diphosphate |
|---|
| Description: | Dianion of thiamine(1+) diphosphate arising from deprotonation of the three OH groups of the diphosphate. |
|---|
|
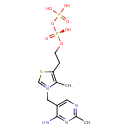
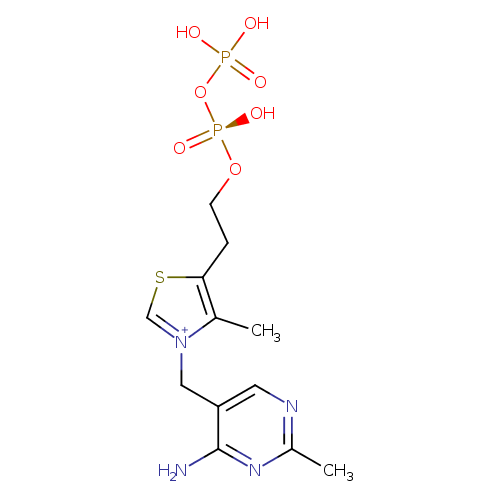
Structure |
|
|---|
| Synonyms: | -
thiamine diphosphate
-
TDP
-
thiamine pyrophosphate
-
TPP
-
thiamin-PPi
-
thiamin pyrophosphate
-
thiamine-PPi
-
ThPP
|
|---|
|
Chemical Formula: |
C12H16N4O7P2S
|
|---|
| Average Molecular Weight: |
422.29 |
|---|
| Monoisotopic Molecular
Weight: |
425.0449676954 |
|---|
| InChI Key: |
AYEKOFBPNLCAJY-UHFFFAOYSA-L |
|---|
| InChI: |
InChI=1S/C12H18N4O7P2S/c1-8-11(3-4-22-25(20,21)23-24(17,18)19)26-7-16(8)6-10-5-14-9(2)15-12(10)13/h5,7H,3-4,6H2,1-2H3,(H4-,13,14,15,17,18,19,20,21)/p-2 |
|---|
| CAS
number: |
154-87-0 |
|---|
| IUPAC Name: | 3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-5-(2-{[hydroxy(phosphonooxy)phosphoryl]oxy}ethyl)-4-methyl-1,3-thiazol-3-ium |
|---|
|
Traditional IUPAC Name: |
thiamin pyrophosphate |
|---|
| SMILES: | CC1([N+](=CSC(CCOP([O-])(=O)OP([O-])(=O)[O-])=1)CC2(C=NC(C)=NC(N)=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Diazines |
|---|
|
Direct Parent |
Thiamine phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Thiamine-phosphate
- Organic pyrophosphate
- 4,5-disubstituted 1,3-thiazole
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Thiazole
- Azole
- Heteroaromatic compound
- Azacycle
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-0201900000-9ca110d3800e4ebfad43 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00di-0900000000-dad8835a4b26b1df86c4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00di-2900000000-83b0eb52fe05e7097aff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-0000900000-538ee08c2fc9a905eaad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0udi-0309000000-b196fd5684e86907d04e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0udi-0409000000-088cf88d5056ce245770 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-9822000000-011b97ebb9fdcebffc93 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-9200000000-2ecd5ee7eb283c5cd990 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-004i-0001900000-59ea8d394aed62700202 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-00b9-0614900000-08f09a377f03019f4457 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-00di-0911000000-de2b56b98b01c6409b09 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-00di-0900000000-7d75ca5105912446e47b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-00di-2900000000-d632b8688f17a7a01de0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Foulon V, Sniekers M, Huysmans E, Asselberghs S, Mahieu V, Mannaerts GP, Van Veldhoven PP, Casteels M: Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase: a revised pathway for the alpha-oxidation of straight chain fatty acids. J Biol Chem. 2005 Mar 18;280(11):9802-12. Epub 2005 Jan 11. [15644336 ]
- Singleton CK, Martin PR: Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001 May;1(2):197-207. [11899071 ]
- Molina JA, Jimenez-Jimenez FJ, Hernanz A, Fernandez-Vivancos E, Medina S, de Bustos F, Gomez-Escalonilla C, Sayed Y: Cerebrospinal fluid levels of thiamine in patients with Alzheimer's disease. J Neural Transm. 2002 Jul;109(7-8):1035-44. [12111441 ]
- Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D: Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995 May;98(5):485-90. [7733128 ]
- Floridi A, Pupita M, Palmerini CA, Fini C, Alberti Fidanza A: Thiamine pyrophosphate determination in whole blood and erythrocytes by high performance liquid chromatography. Int J Vitam Nutr Res. 1984;54(2-3):165-71. [6500839 ]
- Essama-Tjani JC, Guilland JC, Fuchs F, Lombard M, Richard D: Changes in thiamin, riboflavin, niacin, beta-carotene, vitamins, C, A, D and E status of French Elderly Subjects during the first year of institutionalization. Int J Vitam Nutr Res. 2000 Mar;70(2):54-64. [10804457 ]
- Warnock LG: The measurement of erythrocyte thiamin pyrophosphate by high-performance liquid chromatography. Anal Biochem. 1982 Nov 1;126(2):394-7. [7158773 ]
- Lynch PL, Trimble ER, Young IS: High-performance liquid chromatographic determination of thiamine diphosphate in erythrocytes using internal standard methodology. J Chromatogr B Biomed Sci Appl. 1997 Nov 7;701(1):120-3. [9389346 ]
- Naito E, Ito M, Yokota I, Saijo T, Ogawa Y, Kuroda Y: Diagnosis and molecular analysis of three male patients with thiamine-responsive pyruvate dehydrogenase complex deficiency. J Neurol Sci. 2002 Sep 15;201(1-2):33-7. [12163191 ]
- Baines M: Improved high performance liquid chromatographic determination of thiamin diphosphate in erythrocytes. Clin Chim Acta. 1985 Nov 29;153(1):43-8. [4075519 ]
- Duffy P, Morris H, Neilson G: Thiamin status of a Melanesian population. Am J Clin Nutr. 1981 Aug;34(8):1584-92. [7270482 ]
- Kjosen B, Seim SH: The transketolase assay of thiamine in some diseases. Am J Clin Nutr. 1977 Oct;30(10):1591-6. [910736 ]
- Winston AP, Jamieson CP, Madira W, Gatward NM, Palmer RL: Prevalence of thiamin deficiency in anorexia nervosa. Int J Eat Disord. 2000 Dec;28(4):451-4. [11054793 ]
- Levy S, Herve C, Delacoux E, Erlinger S: Thiamine deficiency in hepatitis C virus and alcohol-related liver diseases. Dig Dis Sci. 2002 Mar;47(3):543-8. [11911339 ]
- Talwar D, Davidson H, Cooney J, St JO'Reilly D: Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000 May;46(5):704-10. [10794754 ]
- Fidanza F, Simonetti MS, Floridi A, Codini M, Fidanza R: Comparison of methods for thiamin and riboflavin nutriture in man. Int J Vitam Nutr Res. 1989;59(1):40-7. [2722424 ]
- Tate JR, Nixon PF: Measurement of Michaelis constant for human erythrocyte transketolase and thiamin diphosphate. Anal Biochem. 1987 Jan;160(1):78-87. [3565758 ]
- Frank T, Bitsch R, Maiwald J, Stein G: High thiamine diphosphate concentrations in erythrocytes can be achieved in dialysis patients by oral administration of benfontiamine. Eur J Clin Pharmacol. 2000 Jun;56(3):251-7. [10952481 ]
- Lavoie J, Butterworth RF: Reduced activities of thiamine-dependent enzymes in brains of alcoholics in the absence of Wernicke's encephalopathy. Alcohol Clin Exp Res. 1995 Aug;19(4):1073-7. [7485819 ]
- Casteels M, Foulon V, Mannaerts GP, Van Veldhoven PP: Alpha-oxidation of 3-methyl-substituted fatty acids and its thiamine dependence. Eur J Biochem. 2003 Apr;270(8):1619-27. [12694175 ]
- Schenk G, Duggleby RG, Nixon PF: Properties and functions of the thiamin diphosphate dependent enzyme transketolase. Int J Biochem Cell Biol. 1998 Dec;30(12):1297-318. [9924800 ]
|
|---|
| Synthesis Reference: |
Zabrodskaya, S. V.; Oparin, D. A.; Ostrovskii, Yu. M. Selective synthesis of thiamine diphosphate. Zhurnal Obshchei Khimii (1989), 59(1), 226-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|