|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB006566 |
|---|
|
Identification |
|---|
| Name: |
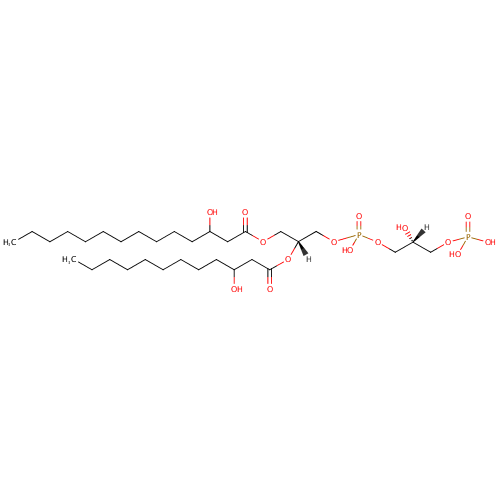
PGP(14:0(3-OH)/12:0(3-OH)) |
|---|
| Description: | PGP(14:0(3-OH)/12:0(3-OH)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(14:0(3-OH)/12:0(3-OH)), in particular, consists of one 3-hydroxytetradecanoyl chain to the C-1 atom, and one 3-hydroxydodecanoyl to the C-2 atom. In Pseudomonas aeruginosa, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase. |
|---|
|
Structure |
|
|---|
| Synonyms: | Not Available |
|---|
|
Chemical Formula: |
C32H64O15P2 |
|---|
| Average Molecular Weight: |
750.797 |
|---|
| Monoisotopic Molecular
Weight: |
750.372045357 |
|---|
| InChI Key: |
HHUPHFAULQQDHE-RAWLKHAVSA-N |
|---|
| InChI: | InChI=1S/C32H64O15P2/c1-3-5-7-9-11-12-14-16-17-19-27(33)21-31(36)43-25-30(26-46-49(41,42)45-24-29(35)23-44-48(38,39)40)47-32(37)22-28(34)20-18-15-13-10-8-6-4-2/h27-30,33-35H,3-26H2,1-2H3,(H,41,42)(H2,38,39,40)/t27?,28?,29-,30-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(2R)-2-hydroxy-3-({hydroxy[(2R)-2-[(3-hydroxydodecanoyl)oxy]-3-[(3-hydroxytetradecanoyl)oxy]propoxy]phosphoryl}oxy)propoxy]phosphonic acid |
|---|
|
Traditional IUPAC Name: |
(2R)-2-hydroxy-3-{[hydroxy((2R)-2-[(3-hydroxydodecanoyl)oxy]-3-[(3-hydroxytetradecanoyl)oxy]propoxy)phosphoryl]oxy}propoxyphosphonic acid |
|---|
| SMILES: | [H][C@@](O)(COP(O)(O)=O)COP(O)(=O)OC[C@@]([H])(COC(=O)CC(O)CCCCCCCCCCC)OC(=O)CC(O)CCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | Not Available |
|---|
|
Kingdom |
Not Available |
|---|
| Super Class | Not Available |
|---|
|
Class |
Not Available |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Not Available |
|---|
| Alternative Parents |
Not Available |
|---|
| Substituents |
Not Available |
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|